Abstract
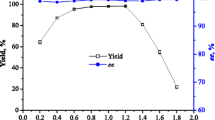
(R)-ethyl-3-hydroxyglutarate with highly optical purity (≥99%) can be used as a novel precursor for synthesis of chiral side chain of rosuvastatin. In this study, a novel synthesis route of (R)-ethyl-3-hydroxyglutarate by whole microorganism cells from racemic ethyl 4-cyano-3-hydroxybutyate was created. A strain ZJB-0910 capable of transforming racemic β-hydroxy aliphatic nitrile was isolated by employing a screening method based on a colorimetric reaction of Co2+ ion with ammonia, and identified as Rhodococcus erythropolis based on its morphology, physiological tests, Biolog, and the 16S rDNA sequence. After cultivation in a sterilized medium with composition of 20 g glucose, 5 g yeast extract, 0.5 g KH2PO4, 0.5 g K2HPO4, 0.2 g MgSO4·7H2O per liter at 30°C and 150 rpm for 48 h, the whole cells of R. erythropolis ZJB-0910 were prepared as a catalyst in (R)-enantioselective hydrolysis of racemic ethyl 4-cyano-3-hydroxybutyate for synthesis of (R)-ethyl-3-hydroxyglutarate, without bearing hydrolase activity for the ester bond of ethyl 4-cyano-3-hydroxybutyate. Under the optimized biotransformation conditions of pH 7.5, 30°C, and 20 mM substrate concentration, (R)-ethyl-3-hydroxyglutarate with 46.2% yield (ee > 99%) was afforded, and its chemical structure was determined by ESI-MS, NMR, and IR. The apparent Michaelis constant K m and maximum rate V max for this biocatalytic reaction were 0.01 M and 85.6 μmol min−1 g−1, respectively.








Similar content being viewed by others
References
Banerjee A, Kaul P, Sharma R, Banerjee UC (2003a) A high-throughput amenable colorimetric assay for enantioselective screening of nitrilase-producing microorganisms using pH sensitive indicators. J Biomol Screen 8:559–565
Banerjee A, Sharma R, Banerjee UC (2003b) A rapid and sensitive fluorometric assay method for the determination of nitrilase activity. Biotechnol Appl Biochem 37:289–293
Bergeron S, Chaplin DA, Edwards JH, Ellis BSW, Hill CL, Holt-Tiffin K, Knight JR, Mahoney T, Osborne AP, Ruecroft G (2006) Nitrilase-catalysed desymmetrisation of 3-hydroxyglutaronitrile: Preparation of a statin side-chain intermediate. Org Process Res Dev 10:661–665
Chen CS, Fujimoto Y, Girdaukas G, Sih CJ (1982) Quantitative analyses of biochemical kinetic resolution of enantiomers. J Am Chem Soc 104:7294–7299
Chen J, Zheng RC, Zheng YG, Shen YC (2009) Microbial transformation of nitriles to high-value acids or amides. Adv Biochem Eng Biotechnol 113:33–77
Chmura A, Shapovalova AA, van Pelt S, van Rantwijk F, Tourova TP, Muyzer G, Sorokin DYu (2008) Utilization of arylaliphatic nitriles by haloalkaliphilic Halomonas nitrilicus sp. nov. isolated from soda soils. Appl Microbiol Biotechnol 81:371–378
Chung CT, Niemela SL, Miller RH (1989) One-step preparation of competent Escherichia coli: transformation and storage of bacterial cells in the same solution. P Natl Acad Sci USA 86:2172–5
Cohen SG, Khedouri E (1961) Requirement for stereospecificity in hydrolysis by α-chymotrypsin. IV. The hydroxyl substituent. Absolute configurations. J Am Chem Soc 83:4228–4233
Davoodi-Dehaghani F, Vosoughi M, Ziaee AA (2010) Biodesulfurization of dibenzothiophene by a newly isolated Rhodococcus erythropolis stratin. Bioresour Technol 101:1102–1105
DeSantis G, Wong K, Farwell B, Chatman K, Zhu Z, Tomlinson G, Huang H, Tan X, Bibbs L, Chen P, Kretz K, Burk MJ (2003) Creation of a productive, highly enantioselective nitrilase through gene site saturation mutagenesis (GSSM). J Am Chem Soc 125:11476–11477
Dong HP, Zheng YG (2010) Quantitative analysis and separation of chiral (S)-ethyl 3-hydroxyglutarate in bioconversion mixtures by LC and TLC. Chromatographia 71:85–89
Frederich MA, Roger B, Robert EK, David DM, Seidman JG, John AS, Kevin S (1999) Short protocols in molecular biology, 4th edn. John Wiley & Sons, pp 2-12. ISBN: 0471250929
Gopalan AS, Sih CJ (1984) Bifunctional chiral synthons via biochemical methods. 5. Preparation of (S)-ethyl hydrogen-3-hydroxyglutarate, key intermediate to (R)-4-amino-3-hydroxybutyric acid and L-carnitine. Tetrahedron Lett 25:5235–5238
Greenberg WA, Varvak A, Hanson SR, Wong K, Huang HJ, Chen P, Burk MJ (2003) Development of an efficient, scalable, aldolase-catalyzed process for enantioselective synthesis of statin intermediates. P Natl Acad Sci USA 101:5788–5793
Hu JG, Wang YJ, Zheng YG, Shen YC (2007) Isolation of glycolonitrile-hydrolyzing micro- organism based on colorimetric reaction. Enzyme Microb Technol 41:244–249
Jacobsen EE, Hoff BH, Moen AR, Anthonsen T (2003) Enantioselective enzymatic preparation of chiral glutaric monocarboxylic acids and amides. J Mol Catal B Enzym 21:55–58
Jin SJ, Zheng RC, Zheng YG, Shen YC (2008) R-enantioselective hydrolysis of 2, 2-dimethylcyclopropanecarboxamide by amidase from a newly isolated strain Brevibacterium epidermidis ZJB-07021. J Appl Microbiol 105:1150–1157
Kim JS, Tiwari MK, Moon HJ, Jeya M, Ramu T, Oh DK, Kim IW, Lee JK (2009) Identification and characterization of a novel nitrilase from Pseudomonas fluorescens Pf-5. Appl Microbiol Biotechnol 83:273–283
Kinfe HH, Frederick VC-J, Bode ML, Mathiba K, Steenkamp PA, Brady D (2009) Enantioselective hydrolysis of β-hydroxy nitriles using the whole cell biocatalyst Rhodococcus rhodochrous ATCC BAA-870. J Mol Catal B Enzym 59:231–236
Kubac D, Cejkova A, Masak J, Jirku V, Lemaire M, Gallienne E, Bolte J, Stloukal R, Martinkova L (2006) Biotransformation of nitriles by Rhodococcus equi A4 immobilized in Lentikats. J Mol Catal B Enzym 39:59–61
Lee SH, Park OJ (2009) Use and production of chiral 3-hydroxy-δ-butyrolactones and structurally related chemicals. Appl Microbiol Biotechnol 84:817–828
Liljeblad A, Kallinen A, Kanerva LT (2009) Biocatalysis in the preparation of the statin side chain. Current Organic Synthesis 6:362–379
Liu ZQ, Sun ZH (2004) Cloning and expression of D-lactonohydrolase cDNA from Fusarium moniliforme in Saccharomyces cerevisiae. Biotechnol Lett 26:1861–1865
Liu YY, Xu JH, Wu HY, Shen D (2004) Integration of purification with immobilization of Candida rugosa lipase for kinetic resolution of racemic ketoprofen. J Biotechnol 110:209–217
Liu ZQ, Sun ZH, Leng Y (2006) Directed evolution and characterization of a novel d-pantonohydrolase from Fusarium moniliforme. J Agric Food Chem 54(16):5823–30
Liu ZQ, Li Y, Ping LF, Xu YY, Cui FJ, Xue YP, Zheng YG (2007) Isolation and identification of a novel Rhodococcus sp ML-0004 producing epoxide hydrolase and optimization of enzyme production. Process Biochem 42(5):889–94
Liu ZQ, Zhang JF, Zheng YG, Shen YC (2008) Improvement of astaxanthin production by a newly isolated Phaffia rhodozyma mutant with low-energy ion beam implantation. J Appl Microbiol 104:861–872
Meseguer-Lloret S, Verdu-Andres J, Molins-Legua C, Campins-Falco P (2005) Determination of ammonia and primary amine compounds and Kjeldahl nitrogen in water samples with a modified Roth's fluorimetric method. Talanta 65:869–875
Moen AR, Hoff BH, Lars K, Anthonsen T, Jacobsen EE (2004) Absolute configurations of monoesters produced by enzyme catalyzed hydrolysis of diethyl 3-hydroxyglutarate. Tetrahedron Asymmetr 15:1551–1554
Müller M (2005) Chemoenzymatic synthesis of building blocks for statin side chains. Angew Chem Int Ed 44:362–365
Öhrlein R, Baisch G (2003) Chemo-enzymatic approach to statin side-chain building blocks. Adv Synth Catal 345:713–715
Tsutamoto T, Yamaji M, Kawahara C, Nishiyama K, Fujii M, Yamamoto T, Horie M (2009) Effect of simvastatin vs. rosuvastatin on adiponectin and haemoglobin A1c levels in patients with non-ischaemic chronic heart failure. Eur J Heart Fail 11:1195–1201
Van Lingen HL, Van de Mortel JKW, Hekking KFW, Van Delft FL (2003) An efficient synthesis of 1-naphthylbis (oxazoline) and exploration of the scope in asymmetric catalysis. Eur J Org Chem 317-324
Wang MX (2005) Enantioselective biotransformations of nitriles in organic synthesis. Top Catal 35:117–130
Wang YS, Xu JM, Zheng RC, Zheng YG, Shen YC (2008) Improvement of amidase production by a newly isolated Delftia tsuruhatensis ZJB-05174 through optimization of culture medium. J Microbiol Biotechnol 18:1932–1937
Wolf LB, Sonke T, Tjen KCMF, Kaptein B (2001) A biocatalytic route to enantiomerically pure unsaturated a-H-a-amino acids. Adv Synth Catal 343:662–674
Wu ZL, Li ZY (2003) Biocatalytic asymmetric hydrolysis of (±)-β-hydroxy nitriles by Rhodococcus sp. CGMCC 0497. J Mol Catal B Enzym 22:105–112
Yazbeck DR, Durao PJ, Xie ZY, Tao JH (2006) A metal ion-based method for the screening of nitrilases. J Mol Catal B Enzym 39:156–159
Yokoyama M, Imai N, Sugai T, Ohta H (1996) Preparation of both enantiomers of methyl 3-benzoyloxypentanoate by enzyme-catalysed hydrolysis of corresponding racemic nitrile and amide. J Mol Catal B Enzym 1:135–141
Zheng RC, Zheng YG, Shen YC (2007) A screening system for active and enantioselective amidase based on its acyl transfer activity. Appl Microbiol Biotechnol 74:256–262
Zheng YG, Chen J, Liu ZQ, Wu MH, Xing LY, Shen YC (2008) Isolation, identification and characterization of Bacillus subtilis ZJB-063, a versatile nitrile-converting bacterium. Appl Microbiol Biotechnol 77:985–993
Zheng YG, Xue YP, Liu ZQ, Zheng RC, Shen YC (2009) Applications of nitrile converting enzymes in the production of fine chemicals. Chin J Biotech 25:1795–1807
Zhu Q, Fan A, Wang YS, Zhu XQ, Wang Z, Wu MH, Zheng YG (2007) Novel sensitive high-throughput screening strategy for nitrilase-producing strains. Appl Environ Microbiol 73:6053–6057
Acknowledgements
This work was supported by the Major Basic Research Development Program of China (973 Program) (No. 2009CB724704), National High Technology Research and Development Program of China (863 Program) (No. 2009AA02Z203), and the Natural Scientific Foundation of Zhejiang (No. Z4090612).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Dong, HP., Liu, ZQ., Zheng, YG. et al. Novel biosynthesis of (R)-ethyl-3-hydroxyglutarate with (R)-enantioselective hydrolysis of racemic ethyl 4-cyano-3-hydroxybutyate by Rhodococcus erythropolis . Appl Microbiol Biotechnol 87, 1335–1345 (2010). https://doi.org/10.1007/s00253-010-2584-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-010-2584-5