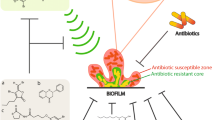
Abstract
Bacteria can switch between planktonic forms (single cells) and biofilms, i.e., bacterial communities growing on solid surfaces and embedded in a matrix of extracellular polymeric substance. Biofilm formation by pathogenic bacteria often results in lower susceptibility to antibiotic treatments and in the development of chronic infections; thus, biofilm formation can be considered an important virulence factor. In recent years, much attention has been directed towards understanding the biology of biofilms and towards searching for inhibitors of biofilm development and of biofilm-related cellular processes. In this report, we review selected examples of target-based screening for anti-biofilm agents: We focus on inhibitors of quorum sensing, possibly the most characterized target for molecules with anti-biofilm activity, and on compounds interfering with the metabolism of the signal molecule cyclic di-GMP metabolism and on inhibitors of DNA and nucleotide biosynthesis, which represent a novel and promising class of biofilm inhibitors. Finally, we discuss the activation of biofilm dispersal as a novel mode of action for anti-biofilm compounds.


Similar content being viewed by others
References
Abdelnour A, Arvidson S, Bremell T, Rydén C, Tarkowski A (1993) The accessory gene regulator (agr) controls Staphylococcus aureus virulence in a murine arthritis model. Infect Immun 61:3879–3885
Abraham WR (2006) Controlling biofilms of gram-positive pathogenic bacteria. Curr Med Chem 13:1509–1524
Allesen-Holm M, Barken KB, Yang L, Klausen M, Webb JS, Kjelleberg S, Molin S, Givskov M, Tolker-Nielsen T (2006) A characterization of DNA release in Pseudomonas aeruginosa cultures and biofilms. Mol Microbiol 59:1114–1128
Amara N, Mashiach R, Amar D, Krief P, Spieser SA, Bottomley MJ, Aharoni A, Meijler MM (2009) Covalent inhibition of bacterial quorum sensing. J Am Chem Soc 131:10610–10619
Anderl JN, Franklin MJ, Stewart PS (2000) Role of antibiotic penetration limitation in Klebsiella pneumoniae biofilm resistance to ampicillin and ciprofloxacin. Antimicrob Agents Chemother 44:1818–1824
Anderson GG, O'Toole GA (2008) Innate and induced resistance mechanisms of bacterial biofilms. Curr Top Microbiol Immunol 322:85–105
Ando T, Suzuki H, Nishimura S, Tanaka T, Hiraishi A, Kikuchi Y (2006) Characterization of extracellular RNAs produced by the marine photosynthetic bacterium Rhodovulum sulfidophilum. J Biochem 139:805–811
Antoniani D, Bocci P, Maciag A, Raffaelli N, Landini P (2010) Monitoring of di-guanylate cyclase activity and of cyclic-di-GMP biosynthesis by whole-cell assays suitable for high-throughput screening of biofilm inhibitors. Appl Microbiol Biotechnol 85:1095–1104
Attila C, Ueda A, Wood TK (2009) 5-Fluorouracil reduces biofilm formation in Escherichia coli K-12 through global regulator AriR as an antivirulence compound. Appl Microbiol Biotechnol 82:525–533
Balaban N, Novick RP (1995) Autocrine regulation of toxin synthesis by Staphylococcus aureus. Proc Natl Acad Sci USA 92:1619–1623
Balaban N, Gov Y, Giacometti A, Cirioni O, Ghiselli R, Mocchegiani F, Orlando F, D'Amato G, Saba V, Scalise G, Bernes S, Mor A (2004) A chimeric peptide composed of a dermaseptin derivative and an RNA III-inhibiting peptide prevents graft-associated infections by antibiotic-resistant staphylococci. Antimicrob Agents Chemother 48:2544–2550
Barber CE, Tang JL, Feng JX, Pan MQ, Wilson TJ, Slater H, Dow JM, Williams P, Daniels MJ (1997) A novel regulatory system required for pathogenicity of Xanthomonas campestris is mediated by a small diffusible signal molecule. Mol Microbiol 24:555–566
Barraud N, Hassett DJ, Hwang SH, Rice SA, Kjelleberg S, Webb JS (2006) Involvement of nitric oxide in biofilm dispersal of Pseudomonas aeruginosa. J Bacteriol 188:7344–7353
Barraud N, Schleheck D, Klebensberger J, Webb JS, Hassett DJ, Rice SA, Kjelleberg S (2009) Nitric oxide signaling in Pseudomonas aeruginosa biofilms mediates phosphodiesterase activity, decreased cyclic diguanosine-5′-monophosphate levels and enhanced dispersal. J Bacteriol 191:7333–7342
Bjarnsholt T, Jensen PØ, Rasmussen TB, Christophersen L, Calum H, Hentzer M, Hougen H, Rygaard J, Moser C, Eberl L, Høiby N, Givskov M (2005) Garlic blocks quorum sensing and promotes rapid clearing of pulmonary Pseudomonas aeruginosa infection. Microbiology 151:3873–3880
Boehm A, Steiner S, Zaehringer F, Casanova A, Hamburger F, Ritz D, Keck W, Ackermann M, Schirmer T, Jenal U (2009) Second messenger signalling governs Escherichia coli biofilm induction upon ribosomal stress. Mol Microbiol 72:1500–1516
Boles BR, Horswill AR (2008) Agr-mediated dispersal of Staphylococcus aureus biofilms. PLoS Pathog 4:e1000052
Boyd A, Chakrabarty AM (1994) Role of alginate lyase in cell detachment of Pseudomonas aeruginosa. Appl Environ Microbiol 60:2355–2359
Burke C, Thomas T, Egan S, Kjelleberg S (2007) The use of functional genomics for the identification of a gene cluster encoding for the biosynthesis of an antifungal tambjamine in the marine bacterium Pseudoalteromonas tunicata. Environ Microbiol 9:814–818
Carté B, Faulkner DJ (1986) Role of secondary metabolites in feeding associations between a predatory nudibranch, two grazing nudibranchs and a bryozoan. J Chem Ecol 12:795–804
Cashel M, Gentry DR, Hernandez VJ, Vinella D (1996) The stringent response. In: Neidhardt FC, Curtiss R, Ingraham JL, Lin ECC, Low KB, Magasanik B, Reznikoff W, Riley M, Schaechter M, Umbarger AE (eds) Escherichia coli and Salmonella typhimurium cellular and molecular biology. American Society of Microbiology, Washington, pp 1458–1496
Ceri H, Olson M, Morck D, Storey D, Read R, Buret A, Olson B (2001) The MBEC Assay System: multiple equivalent biofilms for antibiotic and biocide susceptibility testing. Methods Enzymol 337:377–385
Chan C, Paul R, Samoray D, Amiot NC, Giese B, Jenal U, Schirmer T (2004) Structural basis of activity and allosteric control of diguanylate cyclase. Proc Natl Acad Sci USA 101:17084–17089
Costerton JW, Lewandowski Z, Caldwell DE, Korber DR, Lappin-Scott HM (1995) Microbial biofilms. Annu Rev Microbiol 49:711–745
Cramton SE, Gerke C, Schnell NF, Nichols WW, Götz F (1999) The intercellular adhesion (ica) locus is present in Staphylococcus aureus and is required for biofilm formation. Infect Immun 67:5427–5433
Darouiche RO, Mansouri MD, Gawande PV, Madhyastha S (2009) Antimicrobial and antibiofilm efficacy of triclosan and dispersinB combination. J Antimicrob Chemother 64:88–93
Davies DG, Marques CN (2009) A fatty acid messenger is responsible for inducing dispersion in microbial biofilms. J Bacteriol 191:1393–1403
De N, Pirruccello M, Krasteva PV, Bae N, Raghavan RV, Sondermann H (2008) Phosphorylation-independent regulation of the diguanylate cyclase WspR. PLoS Biol 6:e67
Donelli G, Francolini I, Romoli D, Guaglianone E, Piozzi A, Ragunath C, Kaplan JB (2007) Synergistic activity of dispersinB and cefamandole nafate in inhibition of staphylococcal biofilm growth on polyurethanes. Antimicrob Agents Chemother 51:2733–2740
Dong YH, Gusti AR, Zhang Q, Xu JL, Zhang LH (2002) Identification of quorum-quenching N-acyl homoserine lactonases from Bacillus species. Appl Environ Microbiol 68:1754–1759
Dow JM, Crossman L, Findlay K, He YQ, Feng JX, Tang JL (2003) Biofilm dispersal in Xanthomonas campestris is controlled by cell–cell signaling and is required for full virulence to plants. Proc Natl Acad Sci USA 100:10995–11000
Fuqua C, Winans SC, Greenberg EP (1996) Census and consensus in bacterial ecosystems: the LuxR–LuxI family of quorum-sensing transcriptional regulators. Annu Rev Microbiol 50:727–751
Galperin MY (2004) Bacterial signal transduction network in a genomic perspective. Environ Microbiol 6:552–567
Geske GD, O'Neill JC, Miller DM, Mattmann ME, Blackwell HE (2007) Modulation of bacterial quorum sensing with synthetic ligands: systematic evaluation of N-acylated homoserine lactones in multiple species and new insights into their mechanisms of action. J Am Chem Soc 129:13613–13625
Giacometti A, Cirioni O, Gov Y, Ghiselli R, Del Prete MS, Mocchegiani F, Saba V, Orlando F, Scalise G, Balaban N, Dell'Acqua G (2003) RNA III inhibiting peptide inhibits in vivo biofilm formation by drug-resistant Staphylococcus aureus. Antimicrob Agents Chemother 47:1979–1983
Gjermansen M, Ragas P, Sternberg C, Molin S, Tolker-Nielsen T (2005) Characterization of starvation-induced dispersion in Pseudomonas putida biofilms. Environ Microbiol 7:894–906
Gotoh H, Zhang Y, Dallo SF, Hong S, Kasaraneni N, Weitao T (2008) Pseudomonas aeruginosa, under DNA replication inhibition, tends to form biofilms via Arr. Res Microbiol 159:294–302
Guiton PS, Hung CS, Kline KA, Roth R, Kau AL, Hayes E, Heuser J, Dodson KW, Caparon MG, Hultgren SJ (2009) Contribution of autolysin and Sortase a during Enterococcus faecalis DNA-dependent biofilm development. Infect Immun 77:3626–3638
Hammer BK, Bassler BL (2003) Quorum sensing controls biofilm formation in Vibrio cholerae. Mol Microbiol 50:101–104
Hegde M, Wood TK, Jayaraman A (2009) The neuroendocrine hormone norepinephrine increases Pseudomonas aeruginosa PA14 virulence through the las quorum-sensing pathway. Appl Microbiol Biotechnol 84:763–776
Hentzer M, Riedel K, Rasmussen TB, Heydorn A, Andersen JB, Parsek MR, Rice SA, Eberl L, Molin S, Høiby N, Kjelleberg S, Givskov M (2002) Inhibition of quorum sensing in Pseudomonas aeruginosa biofilm bacteria by a halogenated furanone compound. Microbiology 148:87–102
Hentzer M, Givskov M (2003) Pharmacological inhibition of quorum sensing for the treatment of chronic bacterial infections. J Clin Invest 112:1300–1307
Hentzer M, Wu H, Andersen JB, Riedel K, Rasmussen TB, Bagge N, Kumar N, Schembri MA, Song Z, Kristoffersen P, Manefield M, Costerton JW, Molin S, Eberl L, Steinberg P, Kjelleberg S, Høiby N, Givskov M (2003) Attenuation of Pseudomonas aeruginosa virulence by quorum sensing inhibitors. EMBO J 22:3803–3815
Hoffman LR, D'Argenio DA, MacCoss MJ, Zhang Z, Jones RA, Miller SI (2005) Aminoglycoside antibiotics induce bacterial biofilm formation. Nature 436:1171–1175
Hoffmann N, Lee B, Hentzer M, Rasmussen TB, Song Z, Johansen HK, Givskov M, Høiby N (2007) Azithromycin blocks quorum sensing and alginate polymer formation and increases the sensitivity to serum and stationary-growth-phase killing of Pseudomonas aeruginosa and attenuates chronic P. aeruginosa lung infection in Cftr(−/−) mice. Antimicrob Agents Chemother 51:3677–3687
Holland LM, O'Donnell ST, Ryjenkov DA, Gomelsky L, Slater SR, Fey PD, Gomelsky M, O'Gara JP (2008) A staphylococcal GGDEF domain protein regulates biofilm formation independently of cyclic dimeric GMP. J Bacteriol 190:5178–5189
Horswill AR, Stoodley P, Stewart PS, Parsek MR (2007) The effect of the chemical, biological, and physical environment on quorum sensing in structured microbial communities. Anal Bioanal Chem 387:371–380
Huigens RW 3rd, Ma L, Gambino C, Moeller PD, Basso A, Cavanagh J, Wozniak DJ, Melander C (2008) Control of bacterial biofilms with marine alkaloid derivatives. Mol Biosyst 4:614–621
Iauk L, Lo Bue AM, Milazzo I, Rapisarda A, Blandino G (2003) Antibacterial activity of medicinal plant extracts against periodontopathic bacteria. Phytother Res 17:599–604
Ichimiya T, Takeoka K, Hiramatsu K, Hirai K, Yamasaki T, Nasu M (1996) The influence of azithromycin on the biofilm formation of Pseudomonas aeruginosa in vitro. Chemotherapy 42:186–191
Izano EA, Sadovskaya I, Vinogradov E, Mulks MH, Velliyagounder K, Ragunath C, Kher WB, Ramasubbu N, Jabbouri S, Perry MB, Kaplan JB (2007) Poly-N-acetylglucosamine mediates biofilm formation and antibiotic resistance in Actinobacillus pleuropneumoniae. Microb Pathog 43:1–9
James SG, Holmström C, Kjelleberg S (1996) Purification and characterization of a novel antibacterial protein from the marine bacterium D2. Appl Environ Microbiol 62:2783–2788
Janzon L, Arvidson S (1990) The role of the δ-gene (hld) in the regulation of virulence genes by the accessory gene regulator (agr) in Staphylococcus aureus. EMBO J 9:1391–1399
Junker LM, Clardy J (2007) High-throughput screens for small-molecule inhibitors of Pseudomonas aeruginosa biofilm development. Antimicrob Agents Chemother 51:3582–3590
Kadurugamuwa JL, Beveridge TJ (1995) Virulence factors are released from Pseudomonas aeruginosa in association with membrane vesicles during normal growth and exposure to gentamicin: a novel mechanism of enzyme secretion. J Bacteriol 177:3998–4008
Kaplan JB, Ragunath C, Ramasubbu N, Fine DH (2003) Detachment of Actinobacillus actinomycetemcomitans biofilm cells by an endogenous β-hexosaminidase activity. J Bacteriol 185:4693–4698
Karatan E, Watnick PI (2009) Signals, regulatory networks, and materials that build and break bacterial biofilms. Microbiol Mol Biol Rev 73:310–347
Kiran MD, Adikesavan NV, Cirioni O, Giacometti A, Silvestri C, Scalise G, Ghiselli R, Saba V, Orlando F, Shoham M, Balaban N (2008) Discovery of a quorum-sensing inhibitor of drug-resistant staphylococcal infections by structure-based virtual screening. Mol Pharmacol 73:1578–1586
Kong KF, Vuong C, Otto M (2006) Staphylococcus quorum sensing in biofilm formation and infection. Int J Med Microbiol 296:133–139
König GM, Kehraus S, Seibert SF, Abdel-Lateff A, Müller D (2006) Natural products from marine organisms and their associated microbes. ChemBioChem 7:229–238
Kulasakara H, Lee V, Brencic A, Liberati N, Urbach J, Miyata S, Lee DG, Neely AN, Hyodo M, Hayakawa Y, Asubel FM, Lory S (2006) Analysis of Pseudomonas aeruginosa diguanylate cyclases and phosphodiesterases reveals a role for bis-(3′-5′)-cyclic-GMP in virulence. Proc Natl Acad Sci USA 103:2839–2844
Lewis K (2008) Multidrug tolerance of biofilms and persister cells. Curr Top Microbiol Immunol 322:107–131
Li M, Ni N, Chou HT, Lu CD, Tai PC, Wang B (2008) Structure-based discovery and experimental verification of novel AI-2 quorum sensing inhibitors against Vibrio harveyi. ChemMedChem 3:1242–1249
Li YH, Tang N, Aspiras MB, Lau PC, Lee JH, Ellen RP, Cvitkovitch DG (2002) A quorum-sensing signaling system essential for genetic competence in Streptococcus mutans is involved in biofilm formation. J Bacteriol 184:2699–2708
Lönn-Stensrud J, Landin MA, Benneche T, Petersen FC, Scheie AA (2009) Furanones, potential agents for preventing Staphylococcus epidermidis biofilm infections? J Antimicrob Chemother 63:309–316
Mai-Prochnow A, Lucas-Elio P, Egan S, Thomas T, Webb JS, Sanchez-Amat A, Kjelleberg S (2008) Hydrogen peroxide linked to lysine oxidase activity facilitates biofilm differentiation and dispersal in several gram-negative bacteria. J Bacteriol 190:5493–5501
Manefield M, Rasmussen TB, Henzter M, Andersen JB, Steinberg P, Kjelleberg S, Givskov M (2002) Halogenated furanones inhibit quorum sensing through accelerated LuxR turnover. Microbiology 118:1119–1127
Marketon MM, Glenn SA, Eberhard A, González JE (2003) Quorum sensing controls exopolysaccharide production in Sinorhizobium meliloti. J Bacteriol 185:325–331
McKnight SL, Iglewski BH, Pesci EC (2000) The Pseudomonas quinolone signal regulates rhl quorum sensing in Pseudomonas aeruginosa. J Bacteriol 182:2702–2708
McLennan MK, Ringoir DD, Frirdich E, Svensson SL, Wells DH, Jarrell H, Szymanski CM, Gaynor EC (2008) Campylobacter jejuni biofilms up-regulated in the absence of the stringent response utilize a calcofluor white-reactive polysaccharide. J Bacteriol 190:1097–1107
Miller MB, Bassler BL (2001) Quorum sensing in bacteria. Annu Rev Microbiol l55:165–199
Mizukane R, Hirakata Y, Kaku M, Ishii Y, Furuya N, Ishida K, Koga H, Kohno S, Yamaguchi K (1994) Comparative in vitro exoenzyme-suppressing activities of azithromycin and other macrolide antibiotics against Pseudomonas aeruginosa. Antimicrob Agents Chemother 38:528–533
Morgan R, Kohn D, Hwang SH, Hassett DJ, Sauer K (2006) BdlA, a chemotaxis regulator essential for biofilm dispersion in Pseudomonas aeruginosa. J Bacteriol 188:7335–7343
Müh U, Schuster M, Heim R, Singh A, Olson ER, Greenberg EP (2006) Novel Pseudomonas aeruginosa quorum-sensing inhibitors identified in an ultra-high-throughput screen. Antimicrob Agents Chemother 50:3674–3679
Muto Y, Goto S (1986) Transformation by extracellular DNA produced by Pseudomonas aeruginosa. Microbiol Immunol 30:621–628
Nalca Y, Jänsch L, Bredenbruch F, Geffers R, Buer J, Häussler S (2006) Quorum-sensing antagonistic activities of azithromycin in Pseudomonas aeruginosa PAO1: a global approach. Antimicrob Agents Chemother 50:1680–1688
Njoroge J, Sperandio J (2009) Jamming bacterial communication: new approaches for the treatment of infectious diseases. EMBO Molec Med 1:201–210
Novick RP, Ross HF, Projan SJ, Kornblum J, Kreiswirth B, Moghazeh S (1993) Synthesis of staphylococcal virulence factors is controlled by a regulatory RNA molecule. EMBO J 12:3967–3975
Nucleo E, Steffanoni L, Fugazza G, Migliavacca R, Giacobone E, Navarra A, Pagani L, Landini P (2009) Growth in glucose-based medium and exposure to subinhibitory concentrations of imipenem induce biofilm formation in a multidrug-resistant clinical isolate of Acinetobacter baumannii. BMC Microbiol 9:270
Odenholt I (2001) Pharmacodynamic effects of subinhibitory antibiotic concentrations. Int J Antimicrob Agents 17:1–8
Ozer EA, Pezzulo A, Shih DM, Chun C, Furlong C, Lusis AJ, Greenberg EP, Zabner J (2005) Human and murine paraoxonase 1 are host modulators of Pseudomonas aeruginosa quorum-sensing. FEMS Microbiol Lett 253:29–37
Park SY, Kang HO, Jang HS, Lee JK, Koo BT, Yum DY (2005) Identification of extracellular N-acylhomoserine lactone acylase from a Streptomyces sp. and its application to quorum quenching. Appl Environ Microbiol 71:2632–2641
Paul VJ, Lindquist N, Fenical W (1990) Chemical defences of the tropical ascidian Atapozoa-sp. and its nudibranch predators Nembrotha-spp. Mar Ecol Prog Ser 59:109–118
Persson T, Hansen TH, Rasmussen TB, Skindersø ME, Givskov M, Nielsen J (2005) Rational design and synthesis of new quorum-sensing inhibitors derived from acylated homoserine lactones and natural products from garlic. Org Biomol Chem 3:253–262
Pratten J, Foster SJ, Chan PF, Wilson M, Nair SP (2001) Staphylococcus aureus accessory regulators: expression within biofilms and effect on adhesion. Microbes Infect 3:633–637
Qin Z, Yang L, Qu D, Molin S, Tolker-Nielsen T (2009) Pseudomonas aeruginosa extracellular products inhibit staphylococcal growth, and disrupt established biofilms produced by Staphylococcus epidermidis. Microbiology 155:2148–2156
Rasmussen TB, Skindersoe ME, Bjarnsholt T, Phipps RK, Christensen KB, Jensen PO, Andersen JB, Koch B, Larsen TO, Hentzer M, Eberl L, Hoiby N, Givskov M (2005) Identity and effects of quorum-sensing inhibitors produced by Penicillium species. Microbiology 151:1325–1340
Recsei P, Kreiswirth B, O'Reilly M, Schlievert P, Gruss A, Novick RP (1986) Regulation of exoprotein gene expression in Staphylococcus aureus by agar. Mol Gen Genet 202:58–61
Ren D, Bedzyk LA, Setlow P, England DF, Kjelleberg S, Thomas SM, Ye RW, Wood TK (2004) Differential gene expression to investigate the effect of (5Z)-4-bromo-5-(bromomethylene)-3-butyl-2(5H)-furanone on Bacillus subtilis. Appl Environ Microbiol 70:4941–4949
Richards JJ, Ballard TE, Huigens RW 3rd, Melander C (2008) Synthesis and screening of an oroidin library against Pseudomonas aeruginosa biofilms. Chembiochem 9:1267–1279
Rivardo F, Turner RJ, Allegrone G, Ceri H, Martinotti MG (2009) Anti-adhesion activity of two biosurfactants produced by Bacillus spp. prevents biofilm formation of human bacterial pathogens. Appl Microbiol Biotechnol 83:541–553
Rogers SA, Krayer M, Lindsey JS, Melander C (2009) Tandem dispersion and killing of bacteria from a biofilm. Org Biomol Chem 7:603–606
Sauer K, Cullen MC, Rickard AH, Zeef LA, Davies DG, Gilbert P (2004) Characterization of nutrient-induced dispersion in Pseudomonas aeruginosa PAO1 biofilm. J Bacteriol 186:7312–7326
Simm R, Morr M, Kader A, Nimtiz M, Römling U (2004) GGDEF and EAL domains inversely regulate cyclic di-GMP levels and transition from sessility to motility. Mol Microbiol 53:1123–1134
Smith KM, Bu Y, Suga H (2003) Induction and inhibition of Pseudomonas aeruginosa quorum sensing by synthetic autoinducer analogs. Chem Biol 10:81–89
Smith RS, Iglewski BH (2003) Pseudomonas aeruginosa quorum sensing as a potential antimicrobial target. J Clin Invest 112:1460–1465
Sudarsan N, Lee ER, Weinberg Z, Moy RH, Kim JN, Link KH, Breaker RR (2008) Riboswitches in eubacteria sense the second messenger cyclic di-GMP. Science 321:411–413
Svitil AL, Cashel M, Zyskind JW (1993) Guanosine tetraphosphate inhibits protein synthesis in vivo. A possible protective mechanism for starvation stress in Escherichia coli. J Biol Chem 268:2307–2311
Tamayo R, Pratt JT, Camilli A (2007) Roles of cyclic diguanylate in the regulation of bacterial pathogenesis. Annu Rev Microbiol 61:131–148
Tateda K, Comte R, Pechere JC, Köhler T, Yamaguchi K, Van Delden C (2001) Azithromycin inhibits quorum sensing in Pseudomonas aeruginosa. Antimicrob Agents Chemother 45:1930–1933
Thormann KM, Saville RM, Shukla S, Spormann AM (2005) Induction of rapid detachment in Shewanella oneidensis MR-1 biofilms. J Bacteriol 187:1014–1021
Thormann K, Duttler MS, Saville RM, Hyodo M, Shukla S, Hayakawa Y, Spormann AM (2006) Control of formation and cellular detachment from Shewanella oneidensis MR-1 biofilms by cyclic di-GMP. J Bacteriol 188:2681–2691
Ueda A, Wood TK (2009) Connecting quorum sensing, c-di-GMP, pel polysaccharide, and biofilm formation in Pseudomonas aeruginosa through tyrosine phosphatase TpbA (PA3885). PLoS Pathog 5:e1000483
Ueda A, Attila C, Whiteley M, Wood TK (2009) Uracil influences quorum sensing and biofilm formation in Pseudomonas aeruginosa and fluorouracilnis an antagonist. Microb Biotechnol 2:62–74
Uroz S, Heinonsalo J (2008) Degradation of N-acyl homoserine lactone quorum sensing signal molecules by forest root-associated fungi. FEMS Microbiol Ecol 65:271–278
Vilain S, Pretorius JM, Theron J, Brozel VS (2009) DNA as an adhesin: Bacillus cereus requires extracellular DNA to form biofilms. Appl Environ Microbiol 75:2861–2868
Vuong C, Gerke C, Somerville GA, Fischer ER, Otto M (2003) Quorum-sensing control of biofilm factors in Staphylococcus epidermidis. J Infect Dis 188:706–718
Wagner VE, Gillis RJ, Iglewski BH (2004) Transcriptome analysis of quorum-sensing regulation and virulence factor expression in Pseudomonas aeruginosa. Vaccine 6(22 Suppl 1):S15–20
Wagner VE, Li LL, Isabella VM, Iglewski BH (2007) Analysis of the hierarchy of quorum-sensing regulation in Pseudomonas aeruginosa. Anal Bioanal Chem 387:469–479
Wang LH, He Y, Gao Y, Wu JE, Dong YH, He C, Wang SX, Weng LX, Xu JL, Tay L, Fang RX, Zhang LH (2004a) A bacterial cell–cell communication signal with cross-kingdom structural analogues. Mol Microbiol 51:903–912
Wang X, Preston JF 3rd, Romeo T (2004b) The pgaABCD locus of Escherichia coli promotes the synthesis of a polysaccharide adhesin required for biofilm formation. J Bacteriol 186:2724–2734
Weber H, Pesavento C, Possling A, Tischendorf G, Hengge R (2006) Cyclic-di-GMP-mediated signalling within the sigma network of Escherichia coli. Mol Microbiol 62:1014–1034
Weinhouse H, Sapir S, Amikam D, Shilo Y, Volman G, Ohana P, Benziman M (1997) c-di-GMP-binding protein, a new factor regulating cellulose synthesis in Acetobacter xylinum. FEBS Lett 416:207–211
Whitchurch CB, Tolker-Nielsen T, Ragas PC, Mattick JS (2002) Extracellular DNA required for bacterial biofilm formation. Science 295:1487
Xu L, Li H, Vuong C, Vadyvaloo V, Wang J, Yao Y, Otto M, Gao Q (2006) Role of the luxS quorum-sensing system in biofilm formation and virulence of Staphylococcus epidermidis. Infect Immun 74:488–496
Yang L, Rybtke MT, Jakobsen TH, Hentzer M, Bjarnsholt T, Givskov M, Tolker-Nielsen T (2009) Computer-aided identification of recognized drugs as Pseudomonas aeruginosa quorum-sensing inhibitors. Antimicrob Agents Chemother 53:2432–2443
Yarwood JM, Bartels DJ, Volper EM, Greenberg EP (2004) Quorum sensing in Staphylococcus aureus biofilms. J Bacteriol 186:1838–1850
Zeng Z, Qian L, Cao L, Tan H, Huang Y, Xue X, Shen Y, Zhou S (2008) Virtual screening for novel quorum sensing inhibitors to eradicate biofilm formation of Pseudomonas aeruginosa. Appl Microbiol Biotechnol 79:119–126
Zhu H, Kumar A, Ozkan J, Bandara R, Ding A, Perera I, Steinberg P, Kumar N, Lao W, Griesser SS, Britcher L, Griesser HJ, Willcox MD (2008) Fimbrolide-coated antimicrobial lenses: their in vitro and in vivo effects. Optom Vis Sci 85:292–300
Acknowledgments
Research work in P.L.'s lab was supported by the Italian Foundation for Research on Cystic Fibrosis (project FFC#9/2006, adopted by Gruppo Rocciatori di Belluno) and by the CHEM-PROFARMA-NET Research Program of the Italian Ministry for University and Research (Project RBPR05NWWC_004). RN was funded by a fellowship from the European Community's Seventh Framework Programme, under grant agreement PIEF-GA-2008-219592. JGB acknowledges financial support from the Natural Environment Research Council (NERC) (Awards: NER/T/S/2002/00586/2 and NE/G011206/1.)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Landini, P., Antoniani, D., Burgess, J.G. et al. Molecular mechanisms of compounds affecting bacterial biofilm formation and dispersal. Appl Microbiol Biotechnol 86, 813–823 (2010). https://doi.org/10.1007/s00253-010-2468-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-010-2468-8