Abstract
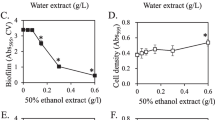
Mushrooms contain large quantities of α-glucans. Shiitake (Lentinula edodes), Japan’s most popular edible mushroom, has been reported to contain about 6% (weight/dried weight) of α-(1,3)-glucan. This glucan is one of the major components of oral biofilm formed by the cariogenic bacteria Streptococcus mutans and Streptococcus sobrinus. We found that extracts from shiitake and other edible mushrooms could reduce preformed biofilms of S. mutans and S. sobrinus in the presence of dextranase. We also investigated the α-glucanase activities of shiitake mushroom extracts and their effects on biofilm formation. The extracts possessed α-glucanase activity and degraded water-insoluble glucans from mutans streptococci. The extracts strongly inhibited the sucrose-dependent formation of biofilms by S. mutans and S. sobrinus in the presence of dextranase. Our results suggest that some components of mushrooms, including α-glucanases, might inhibit the sucrose-induced formation of oral biofilms.






Similar content being viewed by others
References
Ati-Lahsen H, Soler A, Rey M, de La Cruz J, Monte E, Llobell A (2001) An antifungal exo-a-1, 3-glucanase (AGN13.1) from the biocontrol fungus Trichoderma harzianum. Appl Environ Microbiol 67:5833–5839
Dekker N, Speijer D, Grun CH, Berg M, Haan A, Hochstenbach F (2004) Role of the α-glucanase Agn1p in fission-yeast cell separation. Mol Biol Cell 15:3903–3914
Dubois M, Gilles K, Hamilton JK, Rebers PA, Smith F (1956) Colorimetric method for determination of sugars and related substances. Anal Chem 28:350–356
Erhardt FA, Jördening HJ (2007) Immobilization of dextranase from Chaetomium erraticum. J Biotechnol 131:440–447
Fuglsang CC, Berka RM, Wahleithner JA, Kauppinen S, Shuster JR, Rasmussen G, Halkier T, Dalboge H, Henrissat B (2000) Biochemical analysis of recombinant fungal mutanases. J Biol Chem 275:2009–2018
Garcia I, Jimenez D, Martin V, Duran A, Sanchez Y (2005) The alpha-glucanase Agn1p is required for cell separation in Schizosaccharomyces pombe. Biol Cell 97:569–576
Guggenheim B (1970) Enzymatic hydrolysis and structure of water-soluble glucan produced by glucosyltransferases from a strain of Streptococcus mutans. Helv Odontol Acta 14(suppl 5):89
Guggenheim B, Haller R (1972) Purification and properties of an alpha-(1, 3) glucanohydrolase from Trichoderma harzianum. J Dent Res 51:394–402
Hasegawa S, Nordin JH (1969) Enzymes that hydrolyze fungal cell wall polysaccharides. I. Purification and properties of an endo-alpha-D-(1-3)-gucanase from Trichoderma viride. J Biol Chem 244:5460–5470
Hochstenbach F, Klis FM, van den Ende H, van Donselaar E, Peters PJ, Klausner RD (1998) Identification of a putative alpha-glucan synthase essential for cell wall construction and morphogenesis in fission yeast. Proc Natl Acad Sci U S A 95:9161–9166
Johansen C, Falholt P, Gram L (1997) Enzymatic removal and disinfection of bacterial biofilms. Appl Environ Microbiol 63:3724–3728
Khalikova E, Susi P, Korpela T (2005) Microbial dextran-hydrolyzing enzymes: fundamentals and applications. Microbiol Mol Biol Rev 69:306–325
Kim ES, Lee HJ, Bang WG, Choi IG, Kim KH (2009) Functional characterization of a bacterial expansin from Bacillus subtilis for enhanced enzymatic hydrolysis of cellulose. Biotechnol Bioeng 102:1342–1353
Lamont RJ, Jenkinson HF (2000) Adhesion an ecological determinant in the oral cavity. In: Kuramitsu HK, Ellen RP (eds) Oral bacterial ecology: the molecular basis. Horizon, Norfolk, pp 131–168
Lindequist U, Niedermeyer THJ, Jülich WD (2005) The pharmacological potential of mushrooms. CAM 2:285–299
Loesche WJ (1986) Role of Streptococcus mutans in human dental decay. Microbiol Rev 50:353–380
Lynch DJ, Fountain TL, Mazurkiewicz JE, Banas JA (2007) Glucan-binding proteins are essential for shaping Streptococcus mutans biofilm architecture. FEMS Microbiol Lett 268:158–165
Masumoto K, Yamashita K, Yoshida A, Hayashi S, Machida Y, Nagai T (1987) Production and physicochemical properties of water-insoluble glucan from Streptococcus mutans. Chem Pharm Bull 35:3813–3821
Reese AJ, Yoneda A, Breger JA et al (2007) Loss of cell wall alpha(1-3) glucan affects Cryptococcus neoformans from ultrastructure to virulence. Mol Microbiol 63:1385–1398
Sakamoto Y, Irie T, Sato T (2005) Isolation and characterization of a fruiting body specific exo-β-(1, 3)-glucanase-encoding gene, exg1, from Lentinula edodes. Curr Genet 47:244–252
Sakamoto Y, Nagai M, Watanabe H, Nakade K, Takahashi M, Sato T (2006) Lentinula edodes tlg1 encodes a thaumatin-like protein that is involved in lentinan degradation and fruiting body senescence. Plant Physiol 141:793–801
Sakamoto Y, Nakade K, Sato T (2009) Characterization of the post-harvest changes in gene transcription in the gill of the Lentinula edodes fruiting body. Curr Genet 55:409–423
Shcherban TY, Shi J, Durachko DM, Guiltinan MJ, McQueen-Mason SJ, Shich M, Cosgrove DJ (1995) Molecular cloning and sequence analysis of expansins—a highly conserved, multigene family of proteins that mediate cell wall extension in plants. Proc Natl Acad Sci U S A 92:9245–9249
Shida M, Uchida T, Matsuda K (1978) A (1/ar3)-α-D-glucan isolated from the fruit bodies of Lentinus edodes. Carbohydr Res 60:117–127
Shimotsuura I, Kigawa H, Ohdera M, Kuramitsu HK, Nakashima S (2008) Biochemical and molecular characterization of a novel type of mutanase from Paenibacillus sp. Strain RM1: identification of its mutan-binding domain, essential for degradation of Streptococcus mutans biofilms. Appl Environ Microbiol 74:2759–2765
Takehara T, Inoue M (1981) Inhibitory effects of endo-alpha-1, 3-glucanase on glucan film formation and glucan synthesis by the glucosyltransferase of the oral bacterium Streptococcus mutans. Arch Oral Biol 26:217–222
Wei H, Scherer M, Singh A, Liese R, Fisher F (2001) Aspergillus nidulans α-(1, 3)-glucanase (mutanase), mutA, is expressed during sexual development and mobilizes mutan. Fungal Genet Biol 34:217–227
Yamashita Y, Bowen WH, Burne RA, Kuramitsu HK (1993) Role of the Streptococcus mutans gtf genes in caries induction in the specific-pathogen-free rat model. Infect Immun 61:3811–3817
Yano S, Wakayama M, Tachiki T (2006) Cloning and expression of an alpha-1, 3-glucanase gene from Bacillus circulans KA-304: the enzyme participates in protoplast formation of Schizophyllum commune. Biosci Biotechnol Biochem 70:1754–1763
Yoshida A, Ansai T, Takehara T, Kuramitsu HK (2005) LuxS-Based signaling affects Streptococcus mutans biofilm formation. Appl Environ Microbiol 71:2372–2380
Acknowledgements
This study was supported in part by Grants-in-Aid for Scientific Research numbers 21792165 and 20592181, and for High-Tech Research Projects (2009–2010 and 2005–2009, respectively) from the Ministry of Education, Culture, Sports, Science and Technology of Japan.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yano, A., Kikuchi, S., Yamashita, Y. et al. The inhibitory effects of mushroom extracts on sucrose-dependent oral biofilm formation. Appl Microbiol Biotechnol 86, 615–623 (2010). https://doi.org/10.1007/s00253-009-2323-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-009-2323-y