Abstract
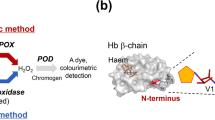
Amadori compounds are ubiquitous in vivo as well as in food and have been implicated in diabetic complications and aging. In recent years, fructosyl amine oxidases (FAOXs) which cleave Amadori products are gaining increasing attention. Until now, however, all FAOXs can only react with small glycated substrates (such as fructosyl amino acids or dipeptides), which has hindered the applications of this new class of enzymes in diagnosis, therapeutics, and detergents. In this study, Aspergillus fumigatus amadoriase II was engineered with the aim to expand its substrate range, using a heat-inducible autolytic vector and fructosyl–polylysine (3–13 lysines) as an intermediate-sized model substrate. After two rounds of directed evolution, a mutant (SII-82) was obtained that showed an 8.78-fold increase in the activity toward fructosyl–polylysine and which also performed several fold better than the wild-type on real gravy stains at concentrations of 10–100 µg/ml (parts per million). Mutational analyses revealed useful clues for altering the substrate-binding pocket. This study suggests that it is possible to manipulate fructosyl amine oxidases to accommodate larger substrates, and that mutant SII-82 might serve as a template for further engineering.




Similar content being viewed by others
References
Aehle W (2004) Enzymes in industry. Wiley, Weinheim
Baynes JW, Thorpe SR (2000) Glycoxidation and lipoxidation in atherogenesis. Free Radical Bio Med 28:1708–1716
Cai Z, Xu WH, Xue R, Lin ZL (2008) Facile, reagentless and in situ release of Escherichia coli intracellular enzymes by heat-inducible autolytic vector for high-throughput screening. Protein Eng Des Sel 21:681–687
Collard F, Zhang J, Nemet I, Qanungo KR, Monnier VM, Yee VC (2008) Crystal structure of the deglycating enzyme fructosamine oxidase (amadoriase II). J Biol Chem 283:27007–27016
Day JF, Thornburg RW, Thorpe SR, Baynes JW (1979) Non-enzymatic glucosylation of rat albumin—studies in vitro and in vivo. J Biol Chem 254:9394–9400
Fujiwara M, Sumitani J, Koga S, Yoshioka I, Kouzuma T, Imamura S, Kawaguchi T, Arai M (2006) Alteration of substrate specificity of fructosyl-amino acid oxidase from Ulocladium sp. JS-103. J Biosci Bioeng 102:241–243
Fujiwara M, Sumitani J, Koga S, Yoshioka I, Kouzuma T, Imamura S, Kawaguchi T, Arai M (2007) Alteration of substrate specificity of fructosyl-amino acid oxidase from Fusarium oxysporum. Appl Microbiol Biotechnol 74:813–819
Garlick RL, Mazer JS (1983) The principal site of nonenzymatic glycosylation of human serum albumin in vivo. J Biol Chem 258:6142–6146
Gerhardinger C, Taneda S, Marion MS, Monnier VM (1994) Isolation, purification, and characterization of an Amadori product binding-protein from a Pseudomonas sp. soil strain. J Biol Chem 269:27297–27302
Heath MM, Rixon KC, Harding JJ (1996) Glycation-induced inactivation of malate dehydrogenase protection by aspirin and a lens molecular chaperone, alpha-crystallin. BBA-Mol Basis Dis 1315:176–184
Hirokawa K, Gomi K, Bakke M, Kajiyama N (2003) Distribution and properties of novel deglycating enzymes for fructosyl peptide in fungi. Arch Microbiol 180:227–231
Hirokawa K, Nakamura K, Kajiyama N (2004) Enzymes used for the determination of HbA1C. FEMS Microbiol Lett 235:157–162
Hirokawa K, Ichiyanagi A, Kajiyama N (2008) Enhancement of thermostability of fungal deglycating enzymes by directed evolution. Appl Microbiol Biotechnol 78:775–781
Horiuchi T, Kurokawa T, Saito N (1989) Purification and properties of fructosyl-amino acid oxidase from Corynebacterium sp. 2-4-1. Agric Biol Chem 53:103–110
Joo H, Lin ZL, Arnold FH (1999) Laboratory evolution of peroxide-mediated cytochrome P450 hydroxylation. Nature 399:670–673
Kobold U, Jeppsson J-O, Dulffer T, Finke A, Hoelzel W, Miedema K (1997) Candidate reference methods for hemoglobin A1C based on peptide mapping. Clin Chem 43:1944–1951
Martins SIFS, Jongen WMF, van Boekel MAJS (2000) A review of Maillard reaction in food and implications to kinetic modelling. Trends Food Sci Technol 11:364–373
Miura S, Ferri S, Tsugawa W, Kim S, Sode K (2008) Development of fructosyl amine oxidase specific to fructosyl valine by site-directed mutagenesis. Protein Eng Des Sel 21:233–239
Mossine VV, Glinsky GV, Feather MS (1994) The preparation and characterization of some Amadori compounds (1-amino-1-deoxy-d-fructose derivatives) derived from a series of aliphatic omega-amino acids. Carbohydr Res 262:257–270
Nakamura K, Masuda I, Kajiyama N (2007) Stabilization of fructosyl peptide oxidase used as diabetic enzyme for clinical diagnosis, involves adding reagent containing chelating reagent of hydroxy carboxylic acid type component, sugar alcohol and amino acid. JP2006325547-A
Nierman WC, Pain A, Anderson MJ, Wortman JR, Kim HS, Arroyo J, Berriman M, Abe K, Archer DB, Bermejo C, Bennett J, Bowyer P, Chen D, Collins M, Coulsen R, Davies R, Dyer PS, Farman M, Fedorova N, Fedorova N (2005) Genomic sequence of the pathogenic and allergenic filamentous fungus Aspergillus fumigatus. Nature 438:1151–1156
Sakaue R, Kajiyama N (2003) Thermostabilization of bacterial fructosyl-amino acid oxidase by directed evolution. Appl Environ Microbiol 69:139–145
Saxena AK, Saxena P, Monnier VM (1996) Purification and characterization of a membrane-bound deglycating enzyme (1-deoxyfructosyl alkyl amino acid oxidase, EC 1.5.3) from a Pseudomonas sp. soil strain. J Biol Chem 271:32803–32809
Shaklai N, Garlick RL, Bunn HF (1984) Nonenzymatic glycosylation of human-serum albumin alters its conformation and function. J Biol Chem 259:3812–3817
Takahashi M, Pischetsrieder M, Monnier VM (1997a) Isolation, purification, and characterization of amadoriase isoenzymes (fructosyl amine-oxygen oxidoreductase EC 1.5.3) from Aspergillus sp. J Biol Chem 272:3437–3443
Takahashi M, Pischetsrieder M, Monnier VM (1997b) Molecular cloning and expression of amadoriase isoenzyme (fructosyl amine:oxygen oxidoreductase, EC 1.5.3) from Aspergillus fumigatus. J Biol Chem 272:12505–12507
Wu XL, Chen SG, Petrash JM, Monnier VM (2002) Alteration of substrate selectivity through mutation of two arginine residues in the binding site of amadoriase II from Aspergillus sp. Biochemistry 41:4453–4458
Xu LH, Li S, Ren C, Cai Z, Lin ZL (2006) Heat-inducible autolytic vector for high-throughput screening. Biotechniques 41:319–322
Yoshida N, Sakai Y, Serata M, Tani Y, Kato N (1995) Distribution and properties of fructosyl amino-acid oxidase in fungi. Appl Environ Microbiol 61:4487–4489
Acknowledgments
The authors would like to thank Prof. Cheng Jin for the A. fumigatus genomic DNA and Pan Li for her technical assistance. This work was supported by grants from the National Basic Research Program of China (2007CB7078004), the National Key Technology R&D Program (2007BAI26B00) of China, and a grant from P&G.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zheng, J., Guan, H., Xu, L. et al. Engineered amadoriase II exhibiting expanded substrate range. Appl Microbiol Biotechnol 86, 607–613 (2010). https://doi.org/10.1007/s00253-009-2319-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-009-2319-7