Abstract
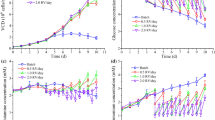
Vero cells growth and rabies production in IPT-AF medium, a property animal-component-free medium are described in this work. Kinetics of cell growth and rabies virus (strain LP 2061) production were first conducted in spinner flasks. Over eight independent experiments, Vero cell growth in IPT-AF medium, on 2 g/l Cytodex 1 was consistent. An average Cd (cell division number) of 3.3 ± 0.4 and a specific growth rate µ of 0.017 ± 0.006 h−1 were achieved. Such performances were comparable to those obtained in serum-containing medium (MEM + 10% FCS). Rabies virus production on Vero cells in IPT-AF medium was also optimised in spinner flasks. The effects of multiplicity of infection (MOI), regulation of glucose level at 1 g/l and cell washing step, were investigated. The highest virus titer was achieved when the cells were infected at an MOI of 0.1; this level was equal to 107 FFU/ml. The step of medium exchange before cell infection can be omitted; nevertheless in this case glucose level should be maintained at 1 g/l to avoid a decrease of specific virus productivity. Process optimisation in a 2-l stirred bioreactor pointed out that the aeration mode was the prominent parameter that affected cell growth in IPT-AF medium and on Cytodex 1 microcarriers. An acceptable level of cell density (cell density level of 1.5 × 106 cells/ml) was achieved when cells were grown in batch mode and using headspace aeration. Nevertheless, this aeration mode is not optimal for large-scale culture. The addition of Pluronic F68 at 0.1% at 24 h post inoculation as well as the switch from surface aeration mode to the sparged mode, 2 days after the start of the culture, had markedly improved cell growth performance. A cell density level of 5.5 × 106 cells/ml was reached when cells were grown in a 2-l bioreactor, on 3 g/l Cytodex 1 in IPT-AF medium and using the recirculation culture mode. Cell infection at an MOI of 0.1 and using perfused culture, resulted in a maximal virus titer of 3.5 × 107 FFU/ml. The activity of the pooled inactivated rabies virus harvests showed a protective activity that meets WHO requirements.








Similar content being viewed by others
References
Butler M, Burgener A, Patrick M, Berry M, Moffatt D, Huzel N, Barnabé N, Coombs K (2000) Application of a serum-free medium for the growth of Vero cells and the production of Reovirus. Biotechnol Prog 16:854–858
Chisti Y (2000) Animal-cell damage in sparged bioreactors. Tibtech 18:420–432
Chu L, Robinson DK (2001) Industrial choices for protein production by large scale cell culture. Curr Opin Biotechnol 12:180–187
Costa WA, Cunha RS, Boltzan VL, Silva ACR, Caporale GMM, Chaves LB, Oselka GWO, Juunqueira DA, Panachao MRI, Dias RA, Takaoka NY (2007) Immunogenicity and safety of a new Vero cell rabies vaccine produced using serum-free medium. Vaccine 25:8140–8145
Doblhoff-Dier O, Stacey G (2000) Cell lines: applications and biosafety. In: Fleming DO, Hunt DL (eds) Biological safety; principals and practices, 3rd edn. ASM, Washington, pp 221–239
Frazatti-Gallina NM, Mourao-Fuches RM, Paoli RL, Silva MLN, Miyaki C, Valentini EJG (2004) Vero-cell rabies vaccine produced using serum-free medium. Vaccine 23:511–517
Frazatti-Gallina NM, Paoli RL, Moura-Fuches RM, Jorge SC, Pereira CA (2001) Higher production of rabies virus in serum-free medium cell cultures on microcarriers. J Biotechnol 92:67–72
Genzel Y, Fischer M, Reichl U (2006a) Serum-free influenza virus production avoiding washing step and medium exchange in large-scale microcarrrier culture. Vaccine 24:3261–3272
Genzel Y, Olmer RM, Schäfer B, Reichl U (2006b) Wave microcarrier cultivation of MDCK cells for influenza virus production in serum containing and serum-free media. Vaccine 24:6074–6087
Hesse F, Wagner R (2000) Developments and improvements in the manufacturing of human therapeutics with mammalian cell cultures. Tibtech 8:173–180
Jayme DW, Smith SR (2000) Media formulation options and manufacturing process controls to safeguard against introduction of animal origin contaminants in animal cell culture. Cytotechnol 33:27–36
Knobel DL, Cleaveland S, Coleman PG, Fèvre EM, Meltzer M, Miranda ME (2005) Reevaluating the burden of rabies in Africa and Asia. Bull WHO 83(5):360–368
Kumar AAP, Mani KR, Palaniappan C, Bhau LNR, Swaminathan K (2005) Purification, potency and immunogenicity analysis of Vero cell culture-derived rabies vaccine: a comparative study of single-step chromatography and zonal centrifuge purification. Microbes Infect 7:1110–1116
Landauer K, Wiederkum S, Dürrschmid M, Klug H, Simic C, Blüml G, Doblhoff-Dier O (2003) Influence of carboxymethyl dextran and ferric citrate on the adhesion of CHO cells on microcarriers. Biotechnol Prog 19:21–29
Liu CC, Lian WC, Butler M, Wu SC (2007) High immunogenic enterovirus 71 strain and its production using serum-free microcarrier Vero cell culture. Vaccine 25:19–24
Lubiniecki AS (1999) Elimination of serum from cell culture medium. Dev Biol Stand 99:15–56
Marcelino I, Sousa MFQ, Vérissimo C, Cunha AE, Carrondo MJT, Alves PM (2006) Process development for the mass production of Ehrlichia ruminantium. Vaccine 24:1716–1725
Merten OW, Kallel H, Manuguerra JC, Tardy-Panit M, Crainic R, Delpeyroux F (1999) The new medium MDSS2N, free of any animal protein supports cell growth and production of various viruses. Cytotechnol 30:191–201
Merten OW, Kierulff JV, Castignolles N, Perrin P (1994) Evaluation of the new serum-free medium (MDSS2) for the production of different biologicals: use of various cell lines. Cytotechnol 14:47–59
Mochizuki M (2004) Growth characteristics of canine pathogenic viruses in MDCK cells cultured in RPMI 1640 medium without animal protein. Vaccine 24:1744–1748
Montagnon B, Fanget B (1996) Purified Vero cell vaccine for humans. In: Meslin FX, Kaplan MM, Koprowski H (eds) Laboratory techniques in rabies. WHO, Geneva, pp 285–289
Morgeaux S, Tordo N, Gontier C, Perrin P (1993) β-propiolactone treatment impairs the biological activity of residual DNA from BHK-21 cells infected with rabies virus. Vaccine 11:82–90
O’Connor J (1998) rtPA is a well-characterized protein. Dev Biol Stand 96:113–121
Petricciani J, Sheets R (2008) An overview of animal cell substrates for biological products. Biologicals 36:359–362
Rourou S, Van der Ark A, Van der Velden T, Kallel H (2007) A microcarrier cell culture process for propagating rabies virus in Vero cells grown in a stirred bioreactor under fully animal component free conditions. Vaccine 25:3879–3889
Rourou S, Van der Ark A, Van der Velden T, Kallel H (2009) Development of an animal component free medium for Vero cells culture. Biotechnol Prog, In press
Silva AC, Delgado I, Sousa MFQ, Carrondo MJT, Alves PM (2008) Scalable culture systems using different cell lines for the production of Peste des Petits ruminants vaccine. Vaccine 26:3305–3311
Smith JS, Yager PA, Baer GM (1973) A rapid tissue culture test for determining rabies neutralizing antibody. In: Meslin FX, Kaplan MM, Koprowski H (eds) Laboratory techniques in rabies. WHO, Geneva, pp 354–357
Tordo N, Bahloul C, Jacob Y, Jallet C, Perrin P, Badrane H (2006) Rabies: epidemiological tendencies and control tools. Dev Biol Stand 125:3–13
Toriniwa H, Komiya T (2007) Japanese encephalitis virus production in Vero cells with serum-free-medium using a novel oscillating bioreactor. Biologicals 35:221–226
Trabelsi K, Rourou S, Loukil H, Majoul S, Kallel H (2005) Comparison of various culture modes for the production of rabies virus by Vero cells grown on microcarriers in a 2-l bioreactor. Enzyme Microb Technol 36:514–519
Trabelsi K, Rourou S, Loukil H, Majoul S, Kallel H (2006) Optimization of virus yield as a strategy to improve rabies vaccine production by Vero cells in a bioreactor. J Biotechnol 121:261–271
Van der Pol L, Tramper J (1998) Shear sensitivity of animal cells from a culture medium perspective. Tibtech 16:323–328
Varani J, Inman DR, Fligiel SEG, Hillegas WJ (1993) Use of recombinant and synthetic peptides as attachment factors for cells on micocarriers. Cytotechnol 13:89–98
Wilbur LA, Aubert MFA (1996) The NIH test for potency. In: Meslin FX, Kaplan MM, Koprowski H (eds) Laboratory techniques in rabies. WHO, Geneva, pp 360–368
World Health Organisation (2005) WHO expert consultation rabies: first report. WHO Technical Report Series, No. 931. Geneva: WHO, p13
World Health Organisation position paper (2007) World Health Organisation 82:425–436
Wu SC, Hung YL (2002) Stationary and microcarrier cell culture process for propagating Japanese Encephalitis Virus. Biotechnol Prog 18:124–128
Wu SC, Liu CC, Lian WC (2004) Optimization of microcarrier cell culture process for the inactivated enterovirus type 71 vaccine development. Vaccine 22:3858–3864
Yokomizo AY, Antoniazzi MM, Galdino PL, JrN A, Jorge SAC, Pereira CA (2004) Rabies virus production in high Vero cell density cultures on macroporous microcarriers. Biotechnol Bioeng 85:506–515
Yuk HI, Lin GB, Ju H, Sifi I, Lam Y, Cortez A, Liebertz D, Berry MJ, Schwartz RM (2006) A serum free Vero production platform for a chimeric virus vaccine candidate. Cytotechnol 51:183–92
Zeng S Bogner, FM, Kunert R, Mueller D, Unterluggauer F (2005) Cell culture process. WO Patent N° 2005069979
Acknowledgement
This work was supported by a grant from MERST (Ministère de la Recherche Scientifique et de la Technologie, Tunisia).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rourou, S., van der Ark, A., Majoul, S. et al. A novel animal-component-free medium for rabies virus production in Vero cells grown on Cytodex 1 microcarriers in a stirred bioreactor. Appl Microbiol Biotechnol 85, 53–63 (2009). https://doi.org/10.1007/s00253-009-2064-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-009-2064-y