Abstract
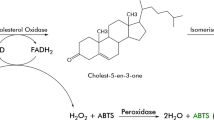
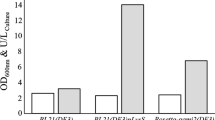
Chromobacterium sp. strain DS-1 produces an extracellular cholesterol oxidase that is very stable at high temperatures and in the presence of organic solvents and detergents. In this study, we cloned and sequenced the structural gene encoding the cholesterol oxidase. The primary translation product was predicted to be 584 amino acid residues. The mature product is composed of 540 amino acid residues. The amino acid sequence of the product showed significant similarity (53–62%) to the cholesterol oxidases from Burkholderia spp. and Pseudomonas aeruginosa. The DNA fragment corresponding to the mature enzyme was subcloned in the pET-21d(+) expression vector and expressed as an active product in Escherichia coli. The cholesterol oxidase produced from the recombinant E. coli was purified to homogeneity. The physicochemical properties were similar to those of native enzyme purified from strain DS-1. K m and V max values of the cholesterol oxidase were estimated from Lineweaver–Burk plots. The V max/K m ratio of the enzyme was higher than those of commercially available cholesterol oxidases. The circular dichroism spectral analysis of the recombinant DS-1 enzyme and Burkholderia cepacia ST-200 cholesterol oxidase showed that the conformational stability of the DS-1 enzyme was higher than that of B. cepacia ST-200 enzyme at higher temperatures.







Similar content being viewed by others
References
Altschul S, Gissh W, Miller W, Mysers E, Lipman D (1990) Basic local alignment search tool. J Mol Biol 215:403–410
Aono R, Doukyu N (1996) Stereospecific oxidation of 3β-hydroxysteroids by persolvent fermentation with Pseudomonas sp. ST-200. Biosci Biotechnol Biochem 60:1146–1151
Aono R, Doukyu N, Kobayashi H, Nakajima H, Horikoshi K (1994) Oxidative bioconversion of cholesterol by Pseudomonas sp. strain ST-200 in a water-organic solvent two-phase system. Appl Environ Microbiol 60:2518–2523
Biellmann J (2001) Resolution of alcohols by cholesterol oxidase from Rhodococcus erythropolis: lack of enantiospecificity for the steroids. Chirality 13:34–39
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Cho HJ, Choi KP, Yamashita M (1995) Introduction and expression of the Streptomyces cholesterol oxidase gene (choA), a potent insecticidal protein active against Boll weevil larvae, into tobacco cells. Appl Microbiol 44:133–138
Coulombe R, Yue KQ, Ghisla S, Vrielink A (2001) Oxygen access to the active site of cholesterol oxidase through a narrow channel is gated by an Arg-Glu pair. J Biol Chem 276:30435–30441
Croteau N, Vrielink A (1996) Crystallization and preliminary X-ray analysis of cholesterol oxidase from Brevibacterium sterolicum containing covalently bound FAD. J Struct Biol 116:317–319
Dieth S, Tritsch D, Biellmann J-F (1995) Resolution of allylic alcohols by cholesterol oxidase isolated from Rhodococcus erythropolis. Tetrahedron Lett 36:2243–2246
Doukyu N, Aono R (1998) Purification of extracellular cholesterol oxidase with high activity in the presence of organic solvents from Pseudomonas sp. ST-200. Appl Environ Microbiol 64:1929–1932
Doukyu N, Aono R (1999) Two moles of O2 consumption and one mole of H2O2 formation with cholesterol peroxidation by cholesterol oxidase from Pseudomonas sp. ST-200. Biochem J 341:621–627
Doukyu N, Aono R (2001) Cloning, sequence analysis and expression of a gene encoding an organic solvent- and detergent-tolerant cholesterol oxidase of Burkholderia cepacia strain ST-200. Appl Microbiol Biotechnol 57:146–152
Doukyu N, Shibata K, Ogino H, Sagermann M (2008) Purification and characterization of Chromobacterium sp. DS-1 cholesterol oxidase with thermal, organic solvent, and detergent tolerance. Appl Microbiol Biotechnol 80:59–70
Fukuyama M, Miyake Y (1979) Purification and some properties of cholesterol oxidase from Schizophyllum commune with covalently bound flavin. J Biochem 85:1183–1193
Inouye Y, Tagnchi K, Fujii A, Ishimaru K, Nakamura S, Nomi R (1982) Purification and characterization of extracellular 3β-hydroxysteroid oxidase produced by Streptoverticillium cholesterolieum. Chem Pharm Bull 30:951–958
Isobe K, Shoji K, Nakanishi Y, Yokoe M, Wakao N (2003) Purification and some properties of cholesterol oxidase stable in detergents from gamma-proteobacterium Y-134. J Biosci Bioeng 95:257–263
Johnson TL, Somkuti GA (1991) Isolation of cholesterol oxidase from Rhodococcus equi ATCC33706. Biotechnol Appl Biochem 13:196–204
Kamei T, Takiguchi Y, Suzuki H, Matsuzaki M, Nakamura S (1978) Purification of 3β-hydroxysteroid oxidase of Streptomyces violascens origin by affinity chromatography on cholesterol. Chem Pharm Bull 26:2799–2804
Kazandjian RZ, Durdich JS, Kilbanov AM (1986) Enzymatic analysis in organic solvents. Biotechnol Bioeng 28:417–421
Laemmli UK (1970) Cleavage of structural proteins during assembly of the head of bacteriophage T4. Nature 224:680–685
Lee S, Rhee H, Tae W, Shin J, Park B (1989) Purification and characterization of cholesterol oxidase from Pseudomonas sp. and taxonomic study of the strain. Appl Microbiol Biotechnol 31:542–546
Liu W-H, Meng M-H, Chen K-S (1988) Purification and some properties of cholesterol oxidases produced by an inducible and a constitutive mutant of Arthrobacter simplex. Agric Biol Chem 52:413–418
MacLachlan J, Wotherspoon ATL, Ansell RO, Brooks CJW (2000) Cholesterol oxidase: sources, physical properties and analytical applications. J Steroid Biochem Mol Biol 72:169–195
Ohta T, Fujishiro K, Yamaguchi K, Tamura Y, Aisaka K, Uwajima T, Hasegawa M (1991) Sequence of gene choB encoding cholesterol oxidase of Brevibacterium sterolicum: comparison with choA of Streptomyces sp. SA-COO. Gene 103:93–96
Pollegioni L, Gadda G, Ambrosius D, Ghisla S, Pilone M (1999) Cholesterol oxidase from Streptomyces hygroscopicus and Brevibacterium sterolicum: effect of surfactants and organic solvents on activity. Biotechnol Appl Biochem 30:27–33
Purcell JP, Greenplate JT, Jennings MG (1993) Cholesterol oxidase: a potent insecticidal protein active against Boll weevil larvae. Biochem Biophys Res Commun 196:1406–1413
Richmond W (1973) Preparation and properties of a cholesterol oxidase from Nocardia sp. and its application to the enzymatic assay of cholesterol in serum. Clin Chem 19:1350–1356
Sambrook J, Maniatis T, Fritsch E (1989) Molecular cloning: a laboratory manual. Cold Spring harbor Laboratory Press, Cold Spring Harbor, NY
Shirokane Y, Nakamura K, Mizusawa K (1977) Purification and some properties of an extracellular 3-hydroxysteroid oxidase produced by Corynebacterium cholesterolicum. J Ferment Technol 55:337–345
Smith AJ, Brooks CJW (1974) Application of cholesterol oxidase in the analysis of steroids. J Chromatog 101:373–378
Smith M, Zahnley J, Pfeifer D, Goff D (1993) Growth and cholesterol oxidation by Mycobacterium species in tween 80 medium. Appl Environ Microbiol 59:1425–1429
Srisawasdi P, Chaichanajarernkul U, Teerakranjana N, Kroll M (2008) Implementation of cellulomonas cholesterol oxidase for total serum cholesterol determination by the endpoint method. J Clin Lab Anal 22:50–58
Tomioka H, Kagawa M, Nakamura S (1976) Some enzymatic properties of 3β-hydroxysteroid oxidase produced by Streptomyces violascens. J Biochem 79:903–915
Uwajima T, Yagi H, Terada O (1974) Properties of crystalline 3β-hydroxysteroid oxidase of Brevibacterium sterolicum. Agric Biol Chem 38:1149–1156
Yang JT, Wu CS, Martinez HM (1986) Calculation of protein conformation from circular dichroism. Methods Enzymol 130:208–269
Acknowledgments
This work was supported in part by the Industrial Technology Research Grant Program in 2005 from New Energy and Industrial Technology Development Organization (NEDO) of Japan, the INOUE ENRYO Memorial Foundation for the Promotion of Sciences, and the Grant for the High Tech Research Center Program organized by the Ministry of Education, Culture, Sports, Science and Technology of Japan, since 2006. We thank Hiromi Kaneko and Kaori Yoshioka for their technical support. We thank Naoki Kajiyama, Kouzou Hirokawa, and Kazuki Shiga (Kikkoman Corp.) for their kind support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Doukyu, N., Shibata, K., Ogino, H. et al. Cloning, sequence analysis, and expression of a gene encoding Chromobacterium sp. DS-1 cholesterol oxidase. Appl Microbiol Biotechnol 82, 479–490 (2009). https://doi.org/10.1007/s00253-008-1775-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-008-1775-9