Abstract
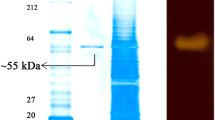
The extremely thermophilic anaerobic archaeon strain, HJ21, was isolated from a deep-sea hydrothermal vent, could produce hyperthermophilic α-amylase, and later was identified as Thermococcus from morphological, biochemical, and physiological characteristics and the 16S ribosomal RNA gene sequence. The extracellular thermostable α-amylase produced by strain HJ21 exhibited maximal activity at pH 5.0. The enzyme was stable in a broad pH range from pH 5.0 to 9.0. The optimal temperature of α-amylase was observed at 95°C. The half-life of the enzyme was 5 h at 90°C. Over 40% and 30% of the enzyme activity remained after incubation at 100°C for 2 and 3 h, respectively. The enzyme did not require Ca2+ for thermostability. This α-amylase gene was cloned, and its nucleotide sequence displayed an open reading frame of 1,374 bp, which encodes a protein of 457 amino acids. Analysis of the deduced amino acid sequence revealed that four homologous regions common in amylases were conserved in the HJ21 α-amylase. The molecular weight of the mature enzyme was calculated to be 51.4 kDa, which correlated well with the size of the purified enzyme as shown by the sodium dodecyl sulfate-polyacrylamide gel electrophoresis.






Similar content being viewed by others
References
Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ (1990) Basic local alignment search tool. J Mol Biol 215:403–410
Atomi H (2005) Recent progress towards the application of hyperthermophiles and their enzymes. Curr Opin Chem Biol 9:166–173
Atomi H, Fukui T, Tanaka T, Kanai T, Morikawa M, Imanaka T (2004) Description of Thermococcus kodakaraensis sp. nov., a well studied hyperthermophilic archaeon previously reported as Pyrococcus sp. KOD1. Archaea 1:263–267
Bae SS, Kim YJ, Yang SH, Lim JK, Jeon JH, Lee HS, Kang SG, Kim SJ, Lee JH (2006) Thermococcus onnurineus sp. nov., a hyperthermophilic archaeon Isolated from a deep-Sea hydrothermal vent area at the Pacmanus Field. J Microbiol Biotechnol 11:1826–1831
Bernhardsdotter ECMJ, Pusey ML, Ng JD, Garriott OK (2004) Cloning and characterization of an α-amylase gene from the hyperthermophilic archaeon Thermococcus thioreducens. Marshall Space Flight Center Database
Brown SH, Costantino HR, Kelly RM (1990) Characterization of amylolytic enzyme activities associated with the hyperthermophilic archaebacterium Pyrococcus furiosus. Appl Environ Microbiol 56:1985–1991
Cambon-Bonavita MA, Lesongeur F, Pignet P, Wery N, Lambert C, Godfroy A, Querellou J, Barbier G (2003) Extremophiles, thermophily section, species description Thermococcus atlanticus sp. nov., a hyperthermophilic archaeon isolated from a deep-sea hydrothermal vent in the Mid-Atlantic Ridge. Extremophiles 7:101–109
Chung YC, Kobayashi T, Kanai H, Akiba T, Kudo T (1995) Purification and properties of extracellular amylase from the hyperthermophilic archaeon Thermococcus profundus DT5432. Appl Environ Microbiol 61:1502–1506
Dong G, Vieille C, Savchenko A, Zeikus JG (1997) Cloning, sequencing, and expression of the gene encoding extracellular a-amylase from Pyrococcus furiosus and biochemical characterization of the recombinant enzyme. Environ Microbiol 63:3569–3576
Jolivet E, Corre E, L, Haridon S, Forterre P, Prieur D (2004) Thermococcus marinus sp. nov., and Thermococcus radiotolerans sp. nov., two hyperthermophilic archaea from deep-sea hydrothermal vents that resist ionizing radiation. Extremophiles 8:219–227
Jone RA, Jermiin LS, Easteal S, Patel BKC, Beacham IR (1999) Amylase and 16S rRNA genes from a hyperthermophilic archaebacterium. J Appl Microbiol 86:93–107
Kuwabara T, Minaba M, Iwayama Y, Inouye I, Nakashima M, Marumo K, Maruyama A, Sugai A, Itoh T, Ishibashi J, Urabe T, Kamekura M (2005) Thermococcus coalescens sp. nov., a cell-fusing hyperthermophilic archaeon from Suiyo Seamount. Int J Syst Evol Microbiol 55:2507–2514
Kuwabara T, Minaba M, Ogi N, Kamekura M (2007) Thermococcus celericrescens sp. nov., a fast-growing and cell-fusing hyperthermophilic archaeon from a deep-sea hydrothermal vent. Int J Syst Evol Microbiol 57:437–443
Kwak YS, Akiba T, Kudo T (1998) Purification and characterization of α-amylase from hyperthermophilic archaeon Thermococcus profundus, which hydrolyzes both α-1, 4 and α-1,6 glucosidic linkages. J Ferment Bioeng 86:363–367
Lee JT, Kanai H, Kobayashi T, Akiba T, Kudo T (1996) Cloning, nucleotide sequence, and hyperexpression of α-amylase Gene from an archaeon, Thermococcus profundus. J Ferment Bioeng 82:432–438
Léveque E, Haye B, Belarbi A (2000a) Cloning and expression of an α-amylase encoding gene from the hyperthermophilic archaebacterium Thermococcus hydrothermalis and biochemical characterization of the recombinant enzyme. FEMS Microbio Lett 186:67–71
Léveque E, Janeček S, Haye B, Belarbi A (2000b) Thermophilic archaeal amylolytic enzymes. Enz Microbiol Technol 26:3–14
Li HF, Chi ZM, Wang XH (2007) Purification and characterization of extracellular amylase from the marine yeast Aureobasidium pullulans N13d and its raw potato starch digestion. Enz Microbiol Technol 40:1006–1012
Nakajima R, Imanaka T, Aiba S (1986) Comparison of amino acid sequences of eleven different alpha -amylases. Appl Microbiol Biotechnol 23:355–360
Nielsen JE, Borchert TV (2000) Protein engineering of bacterial α–amylase. Biochimia et Biophysica Acta 1542:253–274
Pikuta EV, Marsic D, Itoh T, Bej AK, Tang J, Whitman WB, Ng JD, Garriott OK, Hoover RB (2007) Thermococcus thioreducens sp. nov., a novel hyperthermophilic, obligately sulfur-reducing archaeon from a deep-sea hydrothermal vent. Int J Syst Evol Microbiol 57:1612–1618
Rauen HM (1964) Biochemisches Taschenbuch, vol. 2. Springer, Berlin, Germany, pp 90–104
Richardson TH, Tan X, Frey G, Callen W, Cabell M, Lam D, Macomber J, Short JM, Robertson DE, Miller C (2002) A novel, high performance enzyme for starch liquefaction.discovery and optimization of a low pH, thermostable α-amylase. J Biol Chem 277:26501–26507
Tan GH, Gao Y, Shi M, Zhang XY, He SP, Chen ZL, An CC (2005) SiteFinding-PCR: a simple and efficient PCR method for chromosome walking. Nucleic Acids Res 33:2–7
Vieille C, Zeikus GJ (2001) Hyperthermophilic enzymes: sources, uses, and molecular mechanisms for thermostability. Microbiol Mol Biol Rev 65:1–43
Violet M, Meunier JC (1989) Kinetic study of the irreversible thermal denaturation of Bacillus Licheniformis α-amylase. Biochem J 263:665–670
Acknowledgements
The authors would like to thank Mr. Fuchao Li, Ms. Li Chen, and Mr. Jinyu Yang for their technical assistance. This work was financially supported by National Natural Science Foundation of China (40746030), Natural Science Foundation of the Education Department of Jiangsu Province (06KJB550004, 06-A-017), Jiangsu Key Laboratory of Marine Biotechnology (2006HS008), and Science and Technology Department of Lianyungang City (KK06076).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, S., Lu, Z., Lu, M. et al. Identification of archaeon-producing hyperthermophilic α-amylase and characterization of the α-amylase. Appl Microbiol Biotechnol 80, 605–614 (2008). https://doi.org/10.1007/s00253-008-1561-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-008-1561-8