Abstract
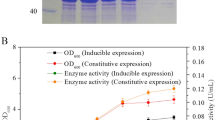
Overexpression and production of the high concentration of hydroxynitrile lyase from cassava (Manihot esculenta (MeHNL, EC 4.1.2.39)) were investigated. Hydroxynitrile lyase is a useful enzyme for the production of optically active cyanohydrin compounds. The production of MeHNL was increased by changing the rare codons of the original sequence of cassava MeHNL. However, most of the produced MeHNL was in the insoluble form. In order to increase the solubility of MeHNL, the effects of the cultivation temperature were investigated. When the cultivation temperature was reduced, the cell yield and the ratio of soluble MeHNL increased significantly. The enzyme activity and yield at low-temperature cultures (17 °C) were 850 times higher than those obtained at the optimum growth temperature of 37 °C. The rate of MeHNL production in the present study was calculated as 3,000 unit/h. Low-temperature cultivation was very effective in improving the productivity of the active form of MeHNL. Unlike the temperature-shift method, low-temperature cultivation has more potential for the large-scale production of MeHNL for the optically active cyanohydrin production.






Similar content being viewed by others
Change history
01 September 2008
The original version of this article unfortunately contained a mistake. The positions of the asterisks of Fig. 1 were shifted. The correct version is given here.
References
Albrecht J, Jansen I, Kula MR (1993) Improved purification of an (R)-oxynitrilase from Linum usitatissimum (flax) and investigation of the substrate range. Biotechnol Appl Biochem 17(Pt 2):191–203
Baedeker M, Schulz GE (1999) Overexpression of a designed 2.2 kb gene of eukaryotic phenylalanine ammonia-lyase in Escherichia coli. FEBS Lett 457:57–60
Bauer S, White MD (1976) Pilot scale exponential growth of Escherichia coli W to high cell concentration with temperature variation. Biotechnol Bioeng 18:839–846
Forster S, Roos J, Effenberger F, Wajant H, Sprauer A (1996) The first hydroxynitrile lyase and its application in the synthesis of (S)-cyanohydrins. Angew Chem Int Ed Engl 35:437–439
Hasslacher M, Schall M, Hayn M, Bona R, Rumbold K, Luckl J, Griengl H, Kohlwein SD, Schwab H (1997) High-level intracellular expression of hydroxynitrile lyase from the tropical rubber tree Hevea brasiliensis in microbial hosts. Protein Expr Purif 11:61–71
Hoshino K, Ito K, Masubuchi A, Adachi M, Asakawa T, Watanabe N, Kosaka T, Tanaka Y (2007) Cloning expression, and characterization of male cynomolgus monkey liver aldehyde oxidase. Biol Pharm Bull 30:1191–1198
Hughes J, Carvalho FJ, Hughes MA (1994) Purification, characterization, and cloning of a-hydroxynitrile lyase from cassava (Manihot esculenta Crantz). Arch Biochem Biophys 311:496–502
Hughes J, Lakey JH, Hughes MA (1997) Production and characterization of a plant a-hydroxynitrile lyase in Escherichia coli. Biotechnol Bioeng 53:332–338
Jensen EB, Carlsen S (1990) Production of recombinant human growth hormone in Escherichia coli: expression of different precursors and physiological effects of glucose, acetate, and salts. Biotechnol Bioeng 36:1–11
Kurokawa Y, Yanagi H, Yura T (2001) Overexpression of bacterial protein disulfide isomerase (DsbC) and its modulator (DsbD) markedly enhances periplasmic production of human nerve growth factor in Escherichia coli. J Biol Chem 276:14393–14399
Nishihara K, Kanemori M, Yanagi H, Yura T (2000) Overexpression of trigger factor prevents aggregation of recombinant proteins in Escherichia coli. Appl Environ Microbiol 66:884–889
Sambrook J, Fritsch EF, Maniatis T (1989) Molecular cloning: a laboratory manual,, 2nd edn. Cold Spring Harbor Laboratory, Cold Spring Harbor, NY
Shiloach J, Bauer S (1975) High-yield growth of E. coli at different temperature in a batch scale fermentor. Biotecnol Bioeng 17:227–239
Singh SM, Panda AK (2005) Solubilization and refolding of bacterial inclusion body proteins. J Biosci Bioeng 99:303–310
Takagi JS, Ida N, Tokushige M, Sakamoto H, Shimura Y (1985) Cloning and nucleotide sequence of the aspartase gene of Escherichia coli W. Nucleic Acids Res 13:2063–2074
Wang Y, Wu SL, Hancock WS, Trala R, Kessler M, Taylor AH, Patel PS, Aon JC (2005) Proteomic profiling of Escherichia coli proteins under high cell density fed-batch cultivation with overexpression of phosphogluconolactonase. Biotechnol Prog 21:1401–1411
Yan G, Cheng S, Zhao G, Wu S, Liu Y, Sun W (2003) A single residual replacement improves the folding and stability of recombinant cassava hydroxynitrile lyase in E. coli. Biotechnol Lett 25:1041–1047
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Semba, H., Ichige, E., Imanaka, T. et al. Efficient production of active form of recombinant cassava hydroxynitrile lyase using Escherichia coli in low-temperature culture. Appl Microbiol Biotechnol 79, 563–569 (2008). https://doi.org/10.1007/s00253-008-1464-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-008-1464-8