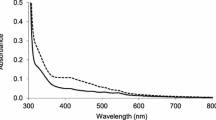
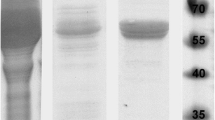
Abstract
A novel bifunctional catalase with an additional phenol oxidase activity was isolated from a thermophilic fungus, Scytalidium thermophilum. This extracellular enzyme was purified ca. 10-fold with 46% yield and was biochemically characterized. The enzyme contains heme and has a molecular weight of 320 kDa with four 80 kDa subunits and an isoelectric point of 5.0. Catalase and phenol oxidase activities were most stable at pH 7.0. The activation energies of catalase and phenol oxidase activities of the enzyme were found to be 2.7 ± 0.2 and 10.1 ± 0.4 kcal/mol, respectively. The pure enzyme can oxidize o-diphenols such as catechol, caffeic acid, and l-DOPA in the absence of hydrogen peroxide and the highest oxidase activity is observed against catechol. No activity is detected against tyrosine and common laccase substrates such as ABTS and syringaldazine with the exception of weak activity with p-hydroquinone. Common catechol oxidase inhibitors, salicylhydroxamic acid and p-coumaric acid, inhibit the oxidase activity. Catechol oxidation activity was also detected in three other catalases tested, from Aspergillus niger, human erythrocyte, and bovine liver, suggesting that this dual catalase-phenol oxidase activity may be a common feature of catalases.





Similar content being viewed by others
References
Allan AC, Walker RL (1988) The selective inhibition of catechol oxidases by salicylhydroxamic acid. Phytochemistry 27(10):3075–3076
Arifoglu N, Ogel ZB (2000) Avicel-adsorbable endoglucanase production by the thermophilic fungus Scytalidium thermophilum type culture Torula thermophila. Enzyme Microb Technol 27:560–569
Arrhenius S (1889) Reaktionsgeschwindigkeit bei der Inversion von Rohrzucker durch Säuren. Z Phys Chem 4:226–248
Bemmann W, Voigt A, Tröger R (1981) Enzymatic studies of the thermophilic hydrocarbon utilizing fungi strains Aspergillus fumigatus and Mucor lusitanicus. Zentralbl Bakteriol Parasitenkd Infektionskr Hyg 136:661–681
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Calera JA, Sanchez-Weatherby J, Lopez-Medrano R, Leal F (2000) Distinctive properties of the catalase B of Aspergillus nidulans. FEBS Lett 475:117–120
Caridis KA, Christakopoulos P, Macris BJ (1991) Simultaneous production of glucose oxidase and catalase by Alternaria alternata. Appl Microbiol Biotechnol 34(6):794–797
Cooney DG, Emerson R (1964) Thermophilic fungi: an account of their biology, activities and classification. Freeman Publishers, San Francisco, USA
Fraaije MW, Roubroeks HP, Hagen WR, Van Berkel WJH (1996) Purification and characterization of an intracellular catalase-peroxidase from Penicilium simplicissimum. Eur J Biochem 235:192–198
Garcia-Molina F, Hiner ANP, Fenoll LG, Rodriguez-Lopez JN, Garcia-Ruiz PA, Garcia-Canovas F, Tudela J (2005) Mushroom tyrosinase: catalase activity, inhibition and suicide inactivation. J Agric Food Chem 53:3702–3709
Gerdemann C, Eicken C, Magrini A, Meyer HE, Rompel A, Spener F, Krebs B (2001) Isoenzymes of Ipomoea batatas catechol oxidase differ in catalase-like activity. Biochem Biophys Acta 1548:94–105
Gessler NN, Sokolov AV, Bykhovsky VY, Belozerskaya TA (2002) Superoxide dismutase and catalase activities in carotenoid-synthesizing fungi Blakeslea trispora and Neurospora crassa fungi in oxidative stress. Appl Biochem Microb 38(3):205–209
Goldberg I, Hochman A (1989) Purification and characterization of a novel type of catalase from the bacterium Klebsiella pneumoniae. Biochem Biophys Acta 991:330–336
Gunata YZ, Sapis JC, Moutounet M (1987) Substrates and aromatic carboxylic acid inhibitors of grape phenol oxidases. Phytochemistry 26(6):1573–1575
Hisada H, Hata Y, Kawato A, Abe Y, Akita O (2005) Cloning and expression analysis of two catalase genes from Aspergillus oryzae. J Biosci Bioeng 99(6):562–568
Hudkova LV, Dehtiar RH, Chumachenko IV, Hulyi MF (1975) Comparative characteristics of catalase from the fungus Penicilium vitale, which is synthesized under different nutritional conditions. Ukr Biohim Z 47(3):342–346
Ikeda-Saito M, Shelley DA, Lu L, Booth KS, Caughey WS, Kimura S (1991) Salicylhydroxamic acid inhibits myeloperoxidase activity. J Biol Chem 266(6):3611–3616
Isobe K, Inoue N, Takamatsu Y, Kamada K, Wakao N (2006) Production of catalase by fungi growing at low pH and high temperature. J Biosci Bioeng 101(1):73–76
Kikuchi-Torii K, Hayashi S, Nakamoto H, Nakamura S (1982) Properties of Aspergillus niger catalase. J Biochem 92(5):1449–1456
Kulys J, Kriauciunas K, Vidziunaite R (2003) Biphasic character of fungal catalases inhibition with hyrdroxylamine in presence of hydrogen peroxide. J Mol Catal B: Enzym 26:79–85
Kwon SI, Anderson AJ (2001) Catalase activities of Phanerochaete chrysosporium are not coordinately produced with ligninolytic metabolism: catalases form a white-rot fungus. Curr Microb 42(1):8–11
Levy E, Eyal Z, Hochman A (1992) Purification and characterization of a catalase–peroxidase from the fungus Septoria tritici. Arch Biochem Biophys 296(1):321–327
Merle PL, Sabourault C, Richier S, Allemand D, Furla P (2007) Catalase characterization and implication in bleaching of a symbiotic sea anemone. Free Radic Biol Med 42:236–246
Montavon P, Kukic KR, Bortlik K (2007) A simple method to measure effective catalase activities: optimization, validation, and application in green coffee. Anal Biochem 306:207–215
Mosavi-Movahedi AA, Wilkinson AE, Jones MN (1987) Characterization of Aspergillus niger catalase. Int J Biol Macromol 9:327–332
Ogel ZB, Yuzugullu Y, Mete S, Bakir U, Kaptan Y, Sutay D, Demir AS (2006) Production, properties and application in biocatalysis of a novel extracellular alkaline phenol oxidase from the thermophilic fungus Scytalidium thermophilum. Appl Microbiol Biotechnol 71(6):853–862
Paris S, Wysong D, Debeaupuis JP, Shibuya K, Philippe B, Diamond RD, Latgé JP (2003) Catalases of Aspergillus fumigatus. Infect Immun 71(6):3551–3562
Pereza L, Hansberg W (2002) Neurospora crassa catalases, singlet oxygen and cell differentiation. Biol Chem 383(3–4):569–575
Pongpom P, Cooper Jr CR, Vanittanakom N (2005) Isolation and characterization of a catalase–peroxidase gene from the pathogenic fungus, Penicillium marneffei. Med Mycol 43(5):403–411
Sanchez-Amat A, Solano F (1997) A pluripotent polyphenol oxidase from the melanogenic marine Alteromonas sp shares catalytic capabilities of tyrosinases and laccases. Biochem Biophys Res Commun 240:787–792
Shibuya K, Paris S, Ando T, Nakayama H, Hatori T, Latgé JP (2006) Catalases of Aspergillus fumigatus and inflammation in Aspergillosis. Jpn J Med Mycol 47(4):249–255
Vainshtein BK, Melik-Adamyan WR, Barynin VV, Vagin AA, Grebenko AI, Borisov VV, Bartels KS, Fita I, Rossmann MG (1986) Three-dimensional structure of catalase from Penicillium vitale at 2.0 A resolution. J Mol Biol 188:49–61
Vetrano AM, Heck DE, Mariano TM, Mishin V, Laskin DL, Laskin JD (2005) Characterization of the oxidase activity in mammalian catalase. J Biol Chem 280(42):35372–35381
Wang H, Tokusige Y, Shinoyama H, Fujii T, Urakami T (1998) Purification and characterization of a thermostable catalase from culture broth of Thermoascus aurantiacus. J Ferment Bioeng 85(2):169–173
Yamazaki S, Morioka C, Itoh S (2004) Kinetic evaluation of catalase and peroxygenase activities of tyrosinase. Biochemistry 43:11546–11553
Zamocky M, Koller F (1999) Understanding the structure and function of catalases: clues from molecular evolution and in vitro mutagenesis. Prog Biophys Mol Biol 72:19–66
Acknowledgements
We would like to thank DPT (AFP-03-04-DPT 2001 K121080), TUBITAK (MISAG-246 and BAYG-2214), and Middle East Technical University (BAP-2006-03-04-01). We thank the Biotechnology and Biological Research Council for funding (SEVP and MJM). We are grateful to J. Keen (LIGHT Laboratories, University of Leeds) for protein sequencing and Chi H. Trinh and Mark A. Smith for their support on this project. We also express our gratitude to the METU Molecular Biology–Biotechnology R&D Center.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sutay Kocabas, D., Bakir, U., Phillips, S.E.V. et al. Purification, characterization, and identification of a novel bifunctional catalase-phenol oxidase from Scytalidium thermophilum . Appl Microbiol Biotechnol 79, 407–415 (2008). https://doi.org/10.1007/s00253-008-1437-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-008-1437-y