Abstract
Conventional processes for lignocellulose-to-organic acid conversion requires pretreatment, enzymatic hydrolysis, and microbial fermentation. In this study, lime-treated wheat straw was hydrolyzed and fermented simultaneously to lactic acid by an enzyme preparation and Bacillus coagulans DSM 2314. Decrease in pH because of lactic acid formation was partially adjusted by automatic addition of the alkaline substrate. After 55 h of incubation, the polymeric glucan, xylan, and arabinan present in the lime-treated straw were hydrolyzed for 55%, 75%, and 80%, respectively. Lactic acid (40.7 g/l) indicated a fermentation efficiency of 81% and a chiral l(+)-lactic acid purity of 97.2%. In total, 711 g lactic acid was produced out of 2,706 g lime-treated straw, representing 43% of the overall theoretical maximum yield. Approximately half of the lactic acid produced was neutralized by fed-batch feeding of lime-treated straw, whereas the remaining half was neutralized during the batch phase with a Ca(OH)2 suspension. Of the lime added during the pretreatment of straw, 61% was used for the neutralization of lactic acid. This is the first demonstration of a process having a combined alkaline pretreatment of lignocellulosic biomass and pH control in fermentation resulting in a significant saving of lime consumption and avoiding the necessity to recycle lime.
Similar content being viewed by others
Introduction
Lactic acid is used throughout the world in manufacturing of food, chemicals, and pharmaceutical products. Recently, there is a lot of interest in biodegradable poly-lactic acid, which is an alternative to petrochemically derived plastic (Drumright et al. 2000). Chiral pure lactic acid is produced commercially by microbial fermentation of the carbohydrates glucose, sucrose, lactose, and starch/maltose derived from feedstocks such as beet sugar, molasses, whey, and barley malt (Narayanan et al. 2004). The choice of feedstock depends on its price, availability, and on the respective costs of lactic acid recovery and purification (Datta et al. 1995; Vaidya et al. 2005).
As an alternative to these traditional feedstocks, lignocellulosic biomass is an inexpensive and widely available renewable carbon source that has no competing food value. Lignocellulose consists primarily of cellulose and hemicellulose; polymers build up of mainly hexose sugars and pentose sugars, which are embedded in a matrix of the phenolic polymer lignin. The main pathway to derive fermentable sugars from lignocellulose is through enzymatic hydrolysis by cellulolytic and hemicellulolytic enzymes. A mechanical and chemical pretreatment of the lignocellulose is required to reduce particle size, to modify and/or to remove the lignin, and with that to enhance the accessibility of the polysaccharides for enzymatic hydrolysis (Claassen et al. 1999). Various chemical pretreatments of biomass have been studied in research and development of lignocellulose-to-ethanol production technology (Mosier et al. 2005). One is the use of lime (calcium hydroxide) at relatively mild temperature conditions (Chang et al. 1998). Lime as a pretreatment agent has promising potential because it is inexpensive, safe, and its use hardly results in sugar degradation products such as furfural and hydroxymethyl furfural. Nevertheless, this alkaline pretreatment features a relatively high pH value (>10) of the treated biomass, and at these pH levels, the activity of common cellulolytic and xylanolytic enzymes, necessary for the depolymerization of (hemi)-cellulose, is negligible low. Therefore, lowering the pH is essential to achieve an efficient enzymatic hydrolysis of the polysaccharides. One approach to remove calcium hydroxide is by washing the lime-treated biomass before enzymatic hydrolysis (Chang et al. 1998); however, this leads to the use of high amounts of water. Another way to lower the pH of the pretreated material is by neutralizing calcium hydroxide with sulfuric acid. Yet, this results in the formation of the low value byproduct gypsum.
As an alternative improvement to these approaches, we propose to use the calcium hydroxide present in lime-treated biomass as neutralizing agent for organic acids produced in microbial fermentation processes. To examine this proposed concept, lime-treated wheat straw (LTWS) was added fed-batch-wise during a simultaneous saccharification and fermentation (SSF) process in a 20-l controlled stirred fermenter containing hydrolytic enzymes and Bacillus coagulans DSM 2314, a thermophilic bacterium capable to convert both hexoses and pentoses homofermentative to l(+)-lactic acid (Otto 2004; Patel et al. 2006). The objective of this research was to evaluate whether high alkaline-treated lignocellulosic biomass (without neutralization) can be used directly in a SSF process by (1) providing a carbon source for enzymatic hydrolysis and fermentation and (2) providing a source of alkali to control the pH in the fermentation process.
Materials and methods
Feedstock and pretreatment
Wheat straw was selected as a lignocellulose model feedstock and was purchased from a farm in the Northeast of The Netherlands. The wheat straw was air dried (89.5% [w/w] dry matter [DM]) and ground through a 2-mm screen. The lime pretreatment was performed by filling two 15-l mixers (Terlet, The Netherlands), both with 1,650 g ground wheat straw, 13 kg tap water, and 165 g calcium hydroxide. This wheat straw suspension was heated and kept at 85°C for 16 h under continuously stirring at 30 rpm. The LTWS suspension was subsequently cooled to 30°C, dehydrated by placing the LTWS in a cotton bag, and pressing the suspension using a manual piston press at pressure up to 9.7 kg/m2. After dehydration, an amount of 11.45 kg LTWS with an average DM content of 27.0% (w/w) and pH 11.8 was obtained and served as substrate for further experiments. The chemical composition of LTWS was determined as described by van den Oever et al. (2003).
Enzyme preparation
The enzyme preparation GC 220 (Genencor-Danisco, Rochester, USA) containing cellulase, cellobiase, and xylanase activity of 116, 215, and 677 U/ml, respectively (Kabel et al. 2006), was used for this study. The preparation had a specific gravity of 1.2 g/ml and contained 4.5 mg/ml glucose, 2.9 mg/ml mannose, and 0.8 mg/ml galactose.
Microorganism and preculture
The bacterium B. coagulans strain DSM 2314 was used as the lactic acid-producing micro-organism. Bacterial cells were maintained in a 10% (w/w) glycerol stock solution and stored at −80°C. Chemicals, unless indicated otherwise, were purchased from Merck (Darmstadt, Germany). Gelrite plates were prepared with a medium containing (per liter): glucose, 10 g; Gelrite, 20 g (Duchefa, Haarlem, The Netherlands); yeast extract, 10 g (Duchefa); (NH4)2HPO4, 2 g; (NH4)2SO4, 3.5 g; Bis–Tris, 10 g (USB, Ohio, USA); MgCl2 6H2O, 0.02 g; and CaCl2.2H2O, 0.1 g. Glucose and Gelrite were dissolved in stock solution A (four times concentrated). The pH of this stock solution was adjusted to 6.4 with 2 M hydrochloric acid and autoclaved for 15 min at 125°C. The remaining nutrients were dissolved in stock solution B (1.33 times concentrated), which was also adjusted to pH 6.4 with 2 M hydrochloric acid but was filter sterilized (cellulose acetate filter with pore size of 0.2 μm, Minisart, Sartorius). After sterilization, the medium was prepared by combining stock solutions A and B and Gelrite plates were poured. The bacteria were cultivated on Gelrite plates for 48 h at 50°C.
An isolated colony was used to inoculate a 100-ml broth with similar composition and preparation as described above but without the addition of Gelrite. The culture was incubated statically for 24 h at 50°C and functioned as the inoculum for a 1,400-ml broth. This culture was incubated also statically for 12 h at 50°C and served as a 10% (v/v) preculture for the SSF experiments.
Simultaneous saccharification and fermentation
The SSF of LTWS was carried out in a 20-l fermenter (Applikon, Schiedam, The Netherlands) with pH and temperature control (biocontroller ADI 1020). At the start of SSF, the fermenter was filled with 6.0 kg tap water and 1,400 g dehydrated LTWS (DM content of 27.0% [w/w]). The following nutrients were then added to the LTWS suspension: yeast extract, 150 g (Duchefa); (NH4)2HPO4, 30 g; (NH4)2SO4, 52.5 g; MgCl2 6H2O, 0.3 g; and CaCl2 2H2O, 1.5 g. The LTWS suspension was then heated to 50°C, and the pH was adjusted to 6.0 with 101 g 3 M sulfuric acid (~30 g H2SO4).
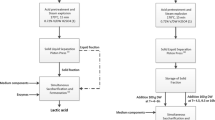
The SSF process of LTWS to lactic acid consisted of three phases: (1) the prehydrolysis phase of preloaded LTWS, (2) the fed-batch phase with automatic feeding of LTWS from a screw feeder, and (3) the batch phase with pH control by a calcium hydroxide suspension and no LTWS feeding. A schematic representation of the experimental setup is shown in Fig. 1. The prehydrolysis was initiated by the addition of 40 ml enzyme preparation (88 mg enzyme/g DM substrate) to the LTWS suspension and was incubated for 2 h at 50°C under continuously stirring at 250 rpm. The fed-batch phase was initiated by the addition of 1,500-ml preculture of B. coagulans DSM 2314 to the fermenter. The lactic acid produced by the bacteria was neutralized by the automatic addition of 8,623 g dehydrated LTWS (DM of 27.0%) to the fermenter through a feeder (K-Tron Soder Feeders, Canada) and was regulated by the pH of the medium, which was set at 6.0. Throughout the fed-batch phase, an amount of 280 ml of enzyme preparation (total enzyme loading of 98 mg/g DM substrate) was added proportional to the LTWS addition rate into the fermenter. During the batch phase, the pH was controlled at 6.0 by the addition of 20.0% (w/v) calcium hydroxide suspension. Samples were withdrawn for DM, substrate, and (by)product analysis.
Analytical methods
For the analysis of monomeric sugars, the fermentation broth samples were centrifuged (3 min at 17,400 × g), and the pH of the supernatant was adjusted to 5.0 with barium carbonate using a pH indicator (Bromophenolblue) followed by filtration of the liquid. The analysis was performed by high-performance anion-exchange chromatography using a Carbopack PA1 column (column temperature of 30°C) and a pulsed amperometric detector (ED50; Dionex, Sunnyvale, CA, USA). Before injection, the system was equilibrated with 25.5 mM NaOH for 10 min at a flow rate of 1.0 ml/min. For the separation of monomeric sugars, at injection, the mobile phase was shifted to deionized water for 30 min. Postcolumn addition of sodium hydroxide was used for detection of the neutral monomeric sugars.
The determination of soluble oligomeric sugars was performed by centrifugation for 5 min at 3,000 rpm (Centaur 2, Beun de Ronde, The Netherlands) of preweighed samples and freeze drying the supernatant overnight. Pellets were weighed and hydrolyzed with sulfuric acid, and neutral monomeric sugars were determined according to the method as described by van den Oever et al. (2003). For the calculations, an average molecular weight of oligomers from glucan and xylan of 166 and 132 g/mol, respectively, were applied, resulting in a hydrolysis factor of 1.08 and 1.14, respectively.
For the analysis of insoluble polymeric sugars, samples of 25 g were centrifuged for 5 min at 3,000 rpm (Centaur 2, Beun de Ronde); the supernatant was removed, and the pellet was washed by resuspension in 25 ml fresh demineralized water followed by a centrifugation step of 5 min at 3,000 rpm (Centaur 2, Beun de Ronde). The sequence of resuspension and centrifugation was repeated three times. After the last removal of the supernatant, the pellets were freeze dried overnight. The pellets were weighed (values used for DM calculation), polymeric material was hydrolyzed with sulfuric acid, and neutral sugars were analyzed according to the method as described by van den Oever et al. (2003). For the calculations, a molecular weight of glucan and xylan of 162 and 132 g/mol, respectively, were applied, resulting in a hydrolysis factor of polymer to monomer of 1.11 and 1.14, respectively.
The analysis of organic acids was performed by high-pressure liquid chromatography according to the procedure described by Maas et al. (2006).
The chiral purity (%) of lactic acid was determined by derivatization of all lactates using methanol, after which both enantiomers of methyl lactate were separated on a chiral gas chromatography column and detected using a flame ionization detector. The chiral purity was expressed as the area of the main enantiomer divided by the sum of areas of both enantiomers.
Calculations
The theoretical maximum lactic acid (LAtheor. max. [g]) production was calculated according the following equation (Eq. 1):
where DMsubstrate = the total dry matter of substrate LTWS (g), F polysacch. = fraction polysaccharides per substrate (g/g), HFmonsacch./polysacch. = hydrolysis factor of polysaccharides, incorporation of water results in 1.11 g hexose from 1.00 g glucan and 1.14 g pentose from xylan and arabinan (g/g), and FF = fermentation factor of 1.00 g lactic acid per gram of monomeric sugar.
The efficiency of the enzymatic hydrolysis (%, w/w) was based on the amount of hydrolyzed polysaccharides (g; calculated by the difference between initial amounts and analyzed insoluble amounts) divided by the amount of polysaccharides (g) initially present in the substrate. The fermentation efficiency (%, w/w) is expressed as the amount of lactic acid produced (g) divided by the amount of monomeric sugars consumed (g) by the bacteria. The overall efficiency of the SSF (%, w/w) was calculated by the amount of lactic acid produced (g) divided by the theoretical maximum amount of lactic acid (g) determined as described in Eq. 1.
Results
Simultaneous saccharification and fermentation of LTWS to lactic acid
The polysaccharide composition of the LTWS consisted mainly of glucan, xylan, and arabinan of 33.0%, 19.0%, and 2.0% (w/w), respectively, whereas the remaining mass constituted of lignin, ash, extractives, and uronic acids. Some of the soluble components in wheat straw were partially removed by the solid/liquid separation (dehydration) of the LTWS. The focus of this study was on the conversion of glucan, xylan, and arabinan, which are the predominant polysaccharides present in LTWS and accounted for 99.8% (w/w) of the total polymeric sugars. Previous work showed that the cellulase preparation GC 220, used for the saccharification of polysaccharides, functioned optimally at 50°C and pH 5.0 (Maas et al., submitted for publication), whereas growth conditions for B. coagulans DSM 2314 were 54°C and pH 6.5 (Otto 2004). In this study, both the enzymatic hydrolysis and the fermentation occurred simultaneously in the same reactor at compromising conditions, which were set at 50°C and pH 6.0.
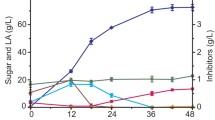
The SSF of LTWS to lactic acid was studied in a 20-l controlled stirred fermenter. Previous results showed that when this process was performed without a prehydrolysis of an initial amount of LTWS, the concentration of monomeric sugars was low and resulted, therefore, in relatively low lactic acid productivity. As a consequence, the fed-batch addition rate of the alkaline substrate to neutralize the produced lactic acid was low (results not shown). To start the fermentation with a substantial initial amount of fermentable sugars (>2 g/l), a prehydrolysis of 378 g LTWS and enzyme preparation (88 mg per g DM LTWS) in approximately 6 l volume at pH 6.0 for 2 h was introduced. This resulted in glucose, xylose, and arabinose concentrations of 2.0, 0.4, and 0.3 g/l, respectively (Fig. 3a).
The second phase (II) was initiated by introducing a 1,500-ml preculture of B. coagulans DSM 2314. A minor amount of lactic acid produced in the preculture caused a slight pH decrease and was automatically neutralized by the addition of LTWS (Fig. 2a,b). After a lag phase of 4 h, the dissolved oxygen concentration decreased rapidly within 1 h from 100% to oxygen-limiting conditions of below 1% (results not shown), and lactic acid production started. At that moment, concentrations of glucose, xylose, and arabinose of 3.3, 0.7, and 0.3 g/l, respectively, were present (Fig. 3a). These sugars were consumed simultaneously where glucose was utilized faster than xylose and arabinose. Simultaneous with the consumption of these monomeric sugars, lactic acid was produced, which was neutralized by the automatic addition of alkaline LTWS. By the addition of alkaline substrate throughout the fed-batch phase, the pH was maintained accurately at 6.0 ± 0.1 (Fig. 2a,b). At the end of phase II, a total amount of 10,023 g dehydrated LTWS (~2,706 g DM LTWS) and 320 ml of enzyme preparation was added to the fermenter. A lactic acid concentration of 20.5 g/l supernatant was detected (Fig. 3b), corresponding to a total of 342 g lactic acid. The chiral L(+) purity of lactic acid was determined at 99.4%, which is similar to that obtained with xylose as the sole carbon source (Otto 2004).
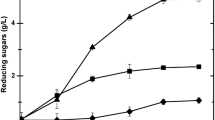
Control of pH (a) during simultaneous saccharification and fermentation of lime-treated wheat straw by commercial enzyme preparation GC 220 and B. coagulans DSM 2314 (b). The areas between the dotted lines represent the prehydrolysis phase (I), the fed-batch phase (II) with pH control by addition of alkaline LTWS and enzymes, and the batch phase (III) with pH control by addition of Ca(OH)2 suspension. Extra enzyme preparation GC220 was added at the times indicated by the arrows
Profiles of glucose (empty square), xylose (empty diamonds), arabinose (empty triangles) (a) and lactic acid (filled diamonds) (b) in simultaneous saccharification and fermentation of lime-treated wheat straw by commercial enzyme preparation GC 220 and B. coagulans DSM 2314. The areas between the dotted lines represent the prehydrolysis phase (I), the fed-batch phase (II), and the batch phase (III). Extra enzyme preparation GC220 was added at the times indicated by the arrows
At the end of phase I, a low acetic acid concentration was detected in the medium, which increased to 1.5 g/l throughout phase II but remained constant during phase III (results not shown). This indicates that acetic acid was most likely not a fermentation product formed by B. coagulans. Acetic acid can be released upon solubilization and hydrolysis of hemicellulose during chemical pretreatment (Palmqvist et al. 1999). By the dehydration procedure of the LTWS, part of the acetic acid was easily separated from the substrate by removing the press water. Apparently, a remaining amount of acetic acid was fed together with the substrate to the fermenter. Furthermore, minor traces of other organic acids such as succinic acid and formic acid (<0.5 g/l) were detected in the fermentation broth.
Phase III was initiated by changing the pH control from the addition of alkaline LTWS to a 20% (w/v) calcium hydroxide suspension. To maintain the pH at 6.0, the addition of calcium hydroxide suspension occurred relatively fast but shifted, however, after a few hours to a lower addition rate indicating a decline of the volumetric lactic acid productivity (Figs. 2b, 3b). To exclude limitation (e.g., by inactivation) of enzymes, an extra dosage of enzyme preparation (80 ml) was added to the fermenter after 23.5 h of incubation. This resulted immediately in a slight acceleration of the calcium hydroxide addition rate indicating an increased lactic acid productivity and limitation of enzymatic activity (Fig. 3b). Nevertheless, after 29.7 h of incubation, a decline of the calcium hydroxide addition rate was observed again. Therefore, a second extra dosage of the enzyme preparation (240 ml) was added and resulted this time in a slight accumulation of glucose and xylose of 1.5 and 1.0 g/l (Fig. 3a), respectively, indicating that microbial conversion instead of enzymatic hydrolysis was rate limiting. After 32 h of incubation, a lactic acid concentration of 37.1 g/l was obtained, with a chiral l(+)-lactic acid purity of 99.4%. Continuation of the SSF process to a total incubation period of 55 h resulted in a slightly increased lactic acid concentration of 40.7 g/l supernatant (~37.8 g lactic acid/kg fermentation broth) with an overall volumetric lactic acid productivity of 0.74 g l−1 h−1. At this stage, a chiral l(+)-lactic acid purity of 97.2% was analyzed. This slight decline in lactic acid purity is possibly a result of infection with other undesired lactic acid-producing microorganisms. Because the substrate used was not sterile and also the chemical pretreatment and fermentation occurred in an open system under nonsterile conditions, microbial contamination throughout the SSF process is possible.
Conversion efficiency
The efficiency of the enzymatic hydrolysis of the polymeric material present in LTWS is shown in Fig. 4. The insoluble polymeric fraction was determined at various time points throughout the SSF experiment. At the end of the prehydrolysis (2 h) of 378 g LTWS, 36% of the insoluble glucan (Fig. 4a), 55% of xylan (Fig. 4b), and 62% of arabinan (Fig. 4c) were converted to soluble saccharides including monomeric sugars and oligomeric sugars. After the fed-batch phase (13 h), 2,706 g LTWS was added and resulted in a conversion of 42% of glucan, 57% of xylan, and 63% of arabinan to products including soluble saccharides and lactic acid. Between 13 and 32 h of incubation, further hydrolysis of the polymeric sugars was observed. However, during the last 23 h of the SSF, minor hydrolysis of the polysaccharides occurred, and this corresponded with the decline in lactic acid productivity during this phase. After 55 h, 398 g of glucan, 130 g of xylan, and 11 g of arabinan was still present as insoluble polymeric material. With these values, the hydrolysis efficiency of the initial glucan, xylan, and arabinan present in LTWS were calculated as 55%, 75%, and 80%, respectively.
Insoluble fraction (empty bars) and hydrolyzed soluble fraction (filled bars) (g; calculated by the difference between initial amounts and analyzed insoluble amounts) of the polysaccharide glucan (a), xylan (b), and arabinan (c) at various time points during the simultaneous saccharification and fermentation of lime-treated wheat straw. The figure represents also the percentage of polysaccharide hydrolyzed into soluble products (filled triangles). The error bars denote the deviation of duplicate analysis
The monomeric sugars, derived from the LTWS, were partly converted to lactic acid (711 g) by B. coagulans and accounted for 81% (w/w) of the theoretical maximum, indicating the formation of other products such as microbial biomass and carbon dioxide. An overall conversion yield of 43% (w/w) of the theoretical maximum was calculated according to Eq. 1. The fate of polysaccharides initially present in LTWS after 55 h of incubation is shown in Table 1. A part of the polysaccharides present in LTWS remained as insoluble polysaccharides (37% w/w), whereas a minor part was converted into soluble oligomeric (5% w/w) and monomeric (3% w/w) sugars. Another part of the initial polysaccharides present in the LTWS was not recovered in the form of saccharides or lactic acid and was therefore ascribed as ‘unaccounted.’
Neutralization of acid by alkaline substrate
The lactic acid produced (342 g) during the fed-batch phase (II) was neutralized with alkaline-pretreated wheat straw. During this phase, an amount of 2,328 g LTWS was added to the fermenter. Together with this substrate, an amount of 230 g calcium hydroxide was added to the fermenter and accounted for a ratio of 0.67 g calcium hydroxide per gram of lactic acid. The lactic acid (369 g) produced during the batch phase (III) was neutralized with 163 g calcium hydroxide resulting in a ratio of 0.44 g lactic acid per gram calcium hydroxide.
Discussion
Lignocellulosic feedstocks are considered as potential attractive substrates for the production of bulk chemicals. Pretreatment of biomass is required to break open the lignocellulosic matrix, and an enzymatic hydrolysis is necessary for the hydrolysis of polymeric carbohydrates. The lime pretreatment has proven to enhance enzymatic digestibility of the polysaccharides present in lignocelluloses (Chang et al. 1998; Kaar and Holtzapple 2000) and results, in comparison to other pretreatment routes, in minor inhibitor formation. However, before the enzymatic hydrolysis, it is essential to adjust the pH to a level optimal for enzymatic activity. In this study, the reduction in pH by washing or neutralization was omitted by using the alkaline character of LTWS to neutralize lactic acid produced by microbial fermentation during a SSF process.
The results showed that the largest part of the polysaccharides in LTWS was converted enzymatically and the resulting sugars were fermented simultaneously to mainly lactic acid by B. coagulans DSM 2314. Between 10 and 30 h of incubation, the bacteria utilized the monomeric sugars, as soon as they appeared in the medium, resulting in relatively low monomeric sugar concentrations (<2 g/l). This indicates that throughout this period, the enzymatic hydrolysis was the rate-controlling step. The highest lactic acid productivity was observed during the fed-batch phase and the initial hours of the batch phase and declined rapidly after approximately 20 h of incubation, as shown in Fig. 3b. An extra addition of enzyme preparation showed a slight improvement of the volumetric lactic acid productivity but shifted within a few hours again to a relatively low production rate. A second extra enzyme addition did not affect the lactic acid productivity significantly (Fig. 3b). This addition of new enzymes resulted in a modest liberation of hemicellulose sugars (xylose, arabinose), but no further hydrolysis of glucan occurred. This shows that the remaining glucan was too recalcitrant or not accessible for further hydrolysis, resulting in decreasing lactic acid productivity. Another possible explanation of the decreased lactic acid productivity is the inhibition of enzymes and/or bacteria by the increasing lactic acid concentration.
A lactic acid concentration of 40.7 g/l supernatant (~37.8 g lactic acid/kg fermentation broth) with a relatively high chiral purity was determined after 55 h of incubation, corresponding to an overall lactic acid yield of 43% of the theoretical maximum. Moreover, the efficiencies of the enzymatic saccharification and the fermentation were both determined. These calculations showed that, based on residue analysis, at the end of the SSF process (55 h), 55% of the glucan, 75% of the xylan, and 80% of the arabinan present in LTWS was enzymatically hydrolyzed, which agree well with previously obtained results from experiments aiming to convert LTWS to ethanol. To improve the yield, it is necessary to decrease the recalcitrance or improve the accessibility of polymeric sugars in the LTWS by optimization of the pretreatment procedure. The concentrations of soluble monosaccharides and oligosaccharides in the medium were relatively low, which can be expected in a SSF process. A fermentation yield of 81% was determined (related to the amount of monosaccharides released from the LTWS) and is slightly better than the results obtained by Otto (2004) who reported the production of 35 g/l lactic acid from 50 g/l xylose as the sole carbon source. Because no other soluble fermentation products were detected, the remaining 19% of the LTWS-derived monomeric sugars were most presumably converted to bacterial biomass and some carbon dioxide during the aerobic part of the fermentation. Several process parameters can be listed for enhancement of the overall lactic acid yield and productivity such as improving the accessibility of polysaccharides by a more severe lime pretreatment, enzyme dosage, type of enzymes, B. coagulans strain, size and growth phase of inoculum, pH gradient in SSF, and in situ product removal of lactic acid. These issues will be subject to further studies.
During the fed-batch phase (II), it was possible to counterbalance the pH decrease caused by lactic acid production by the addition of the alkaline feedstock. This suggests that it is possible to combine lime treatment with the production of other organic acids from lignocellulosic biomass. Throughout this phase, the ratio of calcium hydroxide in LTWS added per produced lactic acid was determined at 0.67 g/g. The theoretical stoichiometric neutralization of 1.00 g lactic acid requires 0.41 g calcium hydroxide. Therefore, only 61% of the calcium hydroxide initially added to the wheat straw was used for lactic acid neutralization. On the other hand, throughout the batch phase (III), an alkaline/acid ratio of 0.44 g/g was calculated corresponding to 93% of the added calcium hydroxide suspension used for lactic acid neutralization. The low efficiency of the calcium hydroxide added with the LTWS for lactic acid neutralization during phase II has three possible explanations. First, part of the calcium hydroxide could have been used during the chemical pretreatment of the wheat straw such as the neutralization of acetic acid or other organic acids and/or irreversible binding to the lignin. Second, the calcium hydroxide might be released slowly from the insoluble wheat straw fibers and could therefore partly have been used for lactic acid neutralization in the fed-batch phase. Finally, besides lactic acid production, other acidification reactions could have contributed to the decrease in pH and therefore the demand of alkaline substrate, for instance, the decrease in pH caused by the consumption of ammonium as the nitrogen source by microorganisms (Guebel et al. 1992).
The results in this paper show that it is possible to use lignocellulosic materials for the production of lactic acid. Lignocellulosic biomass is a relatively inexpensive substrate, and this affects feedstock costs for lactic acid production positively. Nevertheless, in comparison to the traditional relatively ‘clean’ feedstocks with a well-defined composition, using heterogenic lignocellulosic substrates will require a more intensified downstream processing (DSP) to recover and purify the lactic acid from the complex fermentation broth. The costs of feedstock materials and operational costs of the DSP contribute considerably to the overall production costs of lactic acid (Åkerberg and Zacchi 2000). Whether the cost decrease in using lignocellulosic feedstocks outweighs the potential increasing costs of DSP was not analyzed at the moment.
In summary, LTWS was converted into l(+)-lactic acid by B. coagulans throughout a SSF process at a 20-l bench scale. The pentose and hexose sugars derived from the polymeric material were utilized simultaneously by B. coagulans resulting in a final lactic acid concentration of 40.7 g/l supernatant, which accounted for 43% (w/w) of the theoretical yield. To our knowledge, this is the first paper demonstrating a process having a combined alkaline pretreatment of lignocellulosic biomass and pH control in organic acid fermentation resulting in a significant saving of lime consumption and avoiding the necessity to recycle lime.
References
Åkerberg C, Zacchi G (2000) An economic evaluation of the fermentative production of lactic acid from wheat flour. Bioresour Technol 75:119–126
Chang VS, Nagwani M, Holtzapple MT (1998) Lime pretreatment of crop residues bagasse and wheat straw. Appl Biochem Biotechnol 74:135–159
Claassen PAM, van Lier JB, Lopez Contreras AM, van Niel EWJ, Sijtsma L, Stams AJM, de Vries SS, Weusthuis RA (1999) Utilisation of biomass for the supply of energy carriers. Appl Microbiol Biotechnol 52:741–755
Datta R, Tsai S-P, Bonsignore P, Moon S-H, Frank JR (1995) Technological and economic potential of poly(lactic acid) and lactic acid derivatives. FEMS Microbiol Rev 16:221–231
Drumright RE, Gruber PR, Henton DE (2000) Polylactic acid technology. Adv Mater 12:1841–1846
Guebel DV, Cordenos A, Cascone O, Giulietti AM, Nudel C (1992) Influence of the nitrogen source on growth and ethanol production by Pichia stipitis NRRL Y-7124. Biotechnol Lett 14:1193–1198
Kaar WE, Holtzapple MT (2000) Using lime pretreatment to facilitate the enzymic hydrolysis of corn stover. Biomass Bioenergy 18:189–199
Kabel MA, Maarel van den MJEC, Klip G, Voragen AGJ, Schols HA (2006) Standard assays do not predict the efficiency of commercial cellulase preparations towards plant materials. Biotechnol Bioeng 93:56–63
Maas RHW, Bakker RR, Eggink G, Weusthuis RA (2006) Lactic acid production from xylose by the fungus Rhizopus oryzae. Appl Microbiol Biotechnol 72:861–868
Mosier N, Wyman C, Dale B, Elander R, Lee YY, Holtzapple M, Ladisch M (2005) Features of promising technologies for pretreatment of lignocellulosic biomass. Bioresour Technol 96:673–686
Narayanan N, Roychoudhury PK, Srivastava A (2004) L(+) Lactic acid fermentation and its product polymerization. Electron J Biotechnol 7:167–179
Otto R (2004) Preparation of lactic acid from a pentose-containing substrate. Patent WO 2004/063382 A2 1-19
Palmqvist E, Grage H, Meinander NQ, Hahn-Hägerdal B (1999) Main and interaction effects of acetic acid, furfural, and p-hydroxybenzoic acid on growth and ethanol productivity of yeast. Biotechnol Bioeng 63:46–55
Patel MA, Ou MS, Harbrucker R, Aldrich HC, Buszko ML, Ingram LO, Shanmugam KT (2006) Isolation and characterization of acid-tolerant, thermophilic bacteria for effective fermentation of biomass-derived sugars to lactic acid. Appl Environ Microbiol 72:3228–3235
Vaidya AN, Pandey RA, Mudliar S, Suresh Kumar M, Chakrabarti T, Devotta S (2005) Production and recovery of lactic acid for polylactide—an overview. Crit Rev Environ Sci Technol 35:429–467
van den Oever MJA, Elbersen HW, Keijsers ERP, Gosselink RJA, Klerk-Engels de B (2003) Switchgrass (Panicum virgatum L.) as a reinforcing fibre in polypropylene composites. J Mater Sci 38:3697–3707
Acknowledgments
This project is supported with a grant of the Dutch Programme EET (Economy, Ecology, Technology), a joint initiative of the Ministries of Economic Affairs, Education, Culture, and Sciences and of Housing, Spatial Planning and the Environment. The program is run by the EET Programme Office, SenterNovem. Patrick F.N.M. van Doeveren and Jeroen J.C.F. van Bon (AFSG, Wageningen, The Netherlands) are gratefully acknowledged for technical assistance.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Maas, R.H.W., Bakker, R.R., Jansen, M.L.A. et al. Lactic acid production from lime-treated wheat straw by Bacillus coagulans: neutralization of acid by fed-batch addition of alkaline substrate. Appl Microbiol Biotechnol 78, 751–758 (2008). https://doi.org/10.1007/s00253-008-1361-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-008-1361-1