Abstract
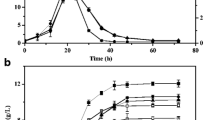
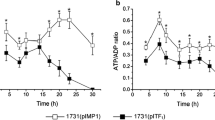
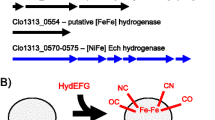
The selective production of acetone and butanol is highly desirable from the viewpoint of biofuel production. We have manipulated the activity level of a hydrogenase for this purpose because hydrogen and solvent production are closely correlated with each other. First, we cloned the hydrogenase gene cluster from Clostridium saccharoperbutylacetonicum strain N1-4 and downregulated its expression using an antisense RNA strategy. The cloned hydrogenase gene cluster contained three adjacent open reading frames, designated hupC, hupB, and hupA. Sequence analysis revealed that HupA could accommodate an H-cluster, which is the catalytic domain of the Fe-hydrogenase. HupB and HupC contained no H-cluster but could accommodate several Fe–S clusters. The hupCBA genes were co-transcribed, and the level of the transcript was maximized in the solventogenic phase. When the antisense RNA of the hupC upstream region (180 bp) was expressed under the bdh (encoding butanol dehydrogenase) promoter, significant reduction of hupC translation was observed, indicating that this antisense RNA is effective in strain N1-4. Production of hydrogen in the antisense transformant increased 3.1-fold. Hydrogen-evolving activity was comparable in both the control and antisense strains, but hydrogen uptake activity significantly decreased in the antisense strain (13% remaining). These results indicate that the HupCBA proteins are involved in hydrogen uptake. Importantly, the level of acetone in the antisense transformant increased 1.6-fold, and butanol production decreased to 75.6% compared to the control strain. Thus, we successfully altered solvent productivity by controlling electron flow in an acetone/butanol-producing Clostridium species.






Similar content being viewed by others
References
Adams MW, Eccleston E, Howard JB (1989) Iron–sulfur clusters of hydrogenase I and hydrogenase II of Clostridium pasteurianum. Proc Natl Acad Sci U S A 86:4932–4936
Andersch W, Bahl H, Gottschalk G (1983) Level of enzymes involved in acetate, butyrate, acetone and butanol formation by Clostridium acetobutylicum. Eur J Appl Microbiol Biotechnol 18:327–332
Chen JS, Blanchard DK (1984) Purification and properties of the H2-oxidizing (uptake) hydrogenase of the N2-fixing anaerobe Clostridium pasteurianum W5. Biochem Biophys Res Commun 122:9–16
Cornillot E, Nair RV, Papoutsakis ET, Soucaille P (1997) The genes for butanol and acetone formation in Clostridium acetobutylicum ATCC 824 reside on a large plasmid whose loss leads to degeneration of the strain. J Bacteriol 179:5442–5447
Datta R, Zeikus JG (1985) Modulation of acetone–butanol–ethanol fermentation by carbon monoxide and organic acids. Appl Environ Microbiol 49:522–529
Desai RP, Papoutsakis ET (1999) Antisense RNA strategies for metabolic engineering of Clostridium acetobutylicum. Appl Environ Microbiol 65:936–945
Doremus MG, Linden JC, Moreira AR (1985) Agitation and pressure effects on acetone–butanol fermentation. Biotechol Bioeng 27:852–860
Dürre P, Fischer RJ, Kuhn A, Lorenz K, Schreiber W, Stürzenhofecker B, Ullmann S, Winzer K, Sauer U (1995) Solventogenic enzymes of Clostridium acetobutylicum: catalytic properties, genetic organization, and transcriptional regulation. FEMS Microbiol Rev 17:251–262
Dürre P, Bohringer M, Nakotte S, Schaffer S, Thormann K, Zickner B (2002) Transcriptional regulation of solventogenesis in Clostridium acetobutylicum. J Mol Microbiol Biotechnol 4:295–300
Fischer RJ, Helms J, Dürre P (1993) Cloning, sequencing, and molecular analysis of the sol operon of Clostridium acetobutylicum, a chromosomal locus involved in solventogenesis. J Bacteriol 175:6959–6969
Gonzalez-Pajuelo M, Meynial-Salles I, Mendes F, Soucaille P, Vasconcelos I (2006) Microbial conversion of glycerol to 1,3-propanediol: physiological comparison of a natural producer, Clostridium butyricum VPI 3266, and an engineered strain, Clostridium acetobutylicum DG1(pSPD5). Appl Environ Microbiol 72:96–101
Gorwa MF, Croux C, Soucaille P (1996) Molecular characterization and transcriptional analysis of the putative hydrogenase gene of Clostridium acetobutylicum ATCC 824. J Bacteriol 178:2668–2675
Hongo M (1965) Bacteriophages of Clostridium saccharoperbutylacetonicum. Part I. Some characteristics of the twelve phages obtained from the abnormally fermented broths. Agric Biol Chem 29:1135–1139
Janssen PJ, Jones DT, Woods DR (1990) Studies on Clostridium acetobutylicum glnA promoters and antisense RNA. Mol Microbiol 4:1575–1583
Johnson JL, Toth J, Santiwatanakul S, Chen JS (1997) Cultures of “Clostridium acetobutylicum” from various collections comprise Clostridium acetobutylicum, Clostridium beijerinckii, and two other distinct types based on DNA-DNA reassociation. Int J Syst Bacteriol 47:420–424
Jones DT, Woods DR (1986) Acetone–butanol fermentation revisited. Microbiol Rev 50:484–524
Junelles AM, Janati-Idrissi R, Petitdemange H, Gay R (1988) Iron effect on acetone–butanol fermentation. Curr Microbiol 17:299–303
Keis S, Shaheen R, Jones DT (2001) Emended descriptions of Clostridium acetobutylicum and Clostridium beijerinckii, and descriptions of Clostridium saccharoperbutylacetonicum sp. nov. and Clostridium saccharobutylicum sp. nov. Int J Syst Evol Microbiol 51:2095–2103
Kim BH, Zeikus JG (1985) Importance of hydrogen metabolism in regulation of solventogenesis by Clostridium acetobutylicum. Dev Ind Microbiol 26:1–14
Kim BH, Bellows P, Datta R, Zeikus JG (1984) Control of carbon and electron flow in Clostridium acetobutylicum fermentations; utilization of carbon monoxide to inhibit hydrogen production and to enhance butanol yields. Appl Environ Microbiol 46:764–770
Kosaka T, Nakayama S, Nakaya K, Yoshino S, Furukawa K (2007) Characterization of the sol operon in butanol-hyperproducing Clostridium saccharoperbutylacetonicum strain N1-4 and its degeneration mechanism. Biosci Biotechnol Biochem 71:58–68
Meyer CL, McLaughlin JK, Papoutsakis ET (1985) The effect of CO on growth and product formation in batch cultures of Clostridium acetobutylicum. Biotechnol Lett 7:37–42
Meyer CL, Roos JW, Papoutsakis ET (1986) Carbon monoxide gassing leads to alcohol production and butyrate uptake without acetone formation in continuous cultures of Clostridium acetobutylicum. Appl Microbiol Biotechnol 24:159–167
Meyer J, Gagnon J (1991) Primary structure of hydrogenase I from Clostridium pasteurianum. Biochemistry 30:9697–9704
Nakayama S, Irie R, Kosaka T, Matsuura K, Yoshino S, Furukawa K (2007) New host–vector system in solvent-producing Clostridium saccharoperbutylacetonicum strain N1-4. J Gen Appl Microbiol 53:53–56
Nölling J, Breton G, Omelchenko MV, Makarova KS, Zeng Q, Gibson R, Lee HM, Dubois J, Qiu D, Hitti J, Wolf YI, Tatusov RL, Sabathe F, Doucette-Stamm L, Soucaille P, Daly MJ, Bennett GN, Koonin EV, Smith DR (2001) Genome sequence and comparative analysis of the solvent-producing bacterium Clostridium acetobutylicum. J Bacteriol 183:4823–4838
Perret S, Maamar H, Belaich JP, Tardif C (2004) Use of antisense RNA to modify the composition of cellulosomes produced by Clostridium cellulolyticum. Mol Microbiol 51:599–607
Peters JW, Lanzilotta WN, Lemon BJ, Seefeldt LC (1998) X-ray structure of the Fe-hydrogenase (CpI) from Clostridium pasteurianum to 1.8 angstrom resolution. Science 282:1853–1858
Peters JW, Szilagyi RK, Naumov A, Douglas T (2006) A radical solution for the biosynthesis of the H-cluster of hydrogenase. FEBS Lett 580:363–367
Petitdemange H, Cherrier C, Bengone JM, Gay R (1977) Study of the NADH and NADPH-ferredoxin oxidoreductase activities in Clostridium acetobutylicum. Can J Microbiol 23:152–160
Ravagnani A, Jennert KC, Steiner E, Grunberg R, Jefferies JR, Wilkinson SR, Young DI, Tidswell EC, Brown DP, Youngman P, Morris JG, Young M (2000) Spo0A directly controls the switch from acid to solvent production in solvent-forming clostridia. Mol Microbiol 37:1172–1185
Santangelo JD, Dürre P, Woods DR (1995) Characterization and expression of the hydrogenase-encoding gene from Clostridium acetobutylicum P262. Microbiology 141:171–180
Scotcher MC, Rudolph FB, Bennett GN (2005) Expression of abrB310 and sinR, and effects of decreased abrB310 expression on the transition from acidogenesis to solventogenesis, in Clostridium acetobutylicum ATCC824. Appl Environ Microbiol 71:1987–1995
Su TM, Lamed R, Lobos JH (1981) Effects of stirring and H2 on ethanol production by thermophilic fermentation. Proceedings of the 2nd World Congress on Chemical Engineering, pp 353–356
Telser J, Benecky MJ, Adams MW, Mortenson LE, Hoffman BM (1987) EPR and electron nuclear double resonance investigation of oxidized hydrogenase II (uptake) from Clostridium pasteurianum W5. Effects of carbon monoxide binding. J Biol Chem 262:6589–94
Thormann K, Feustel L, Lorenz K, Nakotte S, Dürre P (2002) Control of butanol formation in Clostridium acetobutylicum by transcriptional activation. J Bacteriol 184:1966–1973
Toth J, Ismaiel AA, Chen JS (1999) The ald gene, encoding a coenzyme A-acylating aldehyde dehydrogenase, distinguishes Clostridium beijerinckii and two other solvent-producing clostridia from Clostridium acetobutylicum. Appl Environ Microbiol 65:4973–4980
Tummala SB, Junne SG, Papoutsakis ET (2003a) Antisense RNA downregulation of coenzyme A transferase combined with alcohol-aldehyde dehydrogenase overexpression leads to predominantly alcohologenic Clostridium acetobutylicum fermentations. J Bacteriol 185:3644–3653
Tummala SB, Welker NE, Papoutsakis ET (2003b) Design of antisense RNA constructs for downregulation of the acetone formation pathway of Clostridium acetobutylicum. J Bacteriol 185:1923–1934
Vasconcelos I, Girbal L, Soucaille P (1994) Regulation of carbon and electron flow in Clostridium acetobutylicum grown in chemostat culture at neutral pH on mixtures of glucose and glycerol. J Bacteriol 176:1443–1450
Verhagen MF, O’Rourke T, Adams MW (1999) The hyperthermophilic bacterium, Thermotoga maritima, contains an unusually complex iron-hydrogenase: amino acid sequence analyses versus biochemical characterization. Biochim Biophys Acta 1412:212–229
Verhagen MF, O’Rourke T, Adams MW (2001) Heterologous expression and properties of the gamma-subunit of the Fe-hydrogenase from Thermotoga maritima. Biochim Biophys Acta 1505:209–219
Vignais PM, Colbeau A (2004) Molecular biology of microbial hydrogenases. Curr Issues Mol Biol 6:159–188
Vignais PM, Billoud B, Meyer J (2001) Classification and phylogeny of hydrogenases. FEMS Microbiol Rev 25:455–501
Watanabe T, Inoue R, Kimura N, Furukawa K (2000) Versatile transcription of biphenyl catabolic bph operon in Pseudomonas pseudoalcaligenes KF707. J Biol Chem 275:31016–31023
Wilkinson SR, Young DI, Morris JG, Young M (1995) Molecular genetics and the initiation of solventogenesis in Clostridium beijerinckii (formerly Clostridium acetobutylicum) NCIMB 8052. FEMS Microbiol Rev 17:275–285
Yerushalmi L, Volesky B, Szczesny T (1985) Effects of increased hydrogen partial pressure on the acetone–butanol fermentation by Clostridium acetobutylicum. Appl Microbiol Biotechnol 22:103–107
Young M, Minton NP, Staudenbauer WL (1989) Recent advances in the genetics of the clostridia. FEMS Microbiol Rev 63:301–326
Zambrano IC, Kowal AT, Mortenson LE, Adams MW, Johnson MK (1998) Magnetic circular dichroism and electron paramagnetic resonance studies of hydrogenases I and II from Clostridium pasteurianum. J Biol Chem 264:20974–20983
Acknowledgment
This work was supported in part by the New Energy and Industrial Technology Development Organization (NEDO).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nakayama, Si., Kosaka, T., Hirakawa, H. et al. Metabolic engineering for solvent productivity by downregulation of the hydrogenase gene cluster hupCBA in Clostridium saccharoperbutylacetonicum strain N1-4. Appl Microbiol Biotechnol 78, 483–493 (2008). https://doi.org/10.1007/s00253-007-1323-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-007-1323-z