Abstract
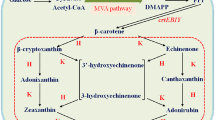
Engineering halophilic bacteria to produce carotenoids is a subject of great scientific and commercial interest, as carotenoids are desirable products used as additives and colorants in the food industry, with β-carotene the most prominent. With this target, we expressed the β-carotene biosynthetic genes crtE, crtY, crtI, and crtB from Pantoea agglomerans and the cDNA encoding isopentenyl pyrophosphate isomerase from Haematococcus pluvialis in the halophilic bacterium Halomonas elongata obtaining a strain able to produce practically pure β-carotene. Reverse transcription-polymerase chain reaction analysis showed crtY, crtI, and crtB heterologous expression in a selected exconjugant of H. elongata. Biosynthesis of β-carotene was dependent on NaCl concentration in the culture medium, with the highest production (560 μg per g of dry weight) in 2% NaCl. On the contrary, no β-carotene was detected in 15% NaCl. Successful construction of the β-carotene biosynthetic pathway in H. elongata opens the possibility of engineering halophilic bacteria for carotenoid production.




Similar content being viewed by others
References
Ausubel FM, Brent R, Kingston RE, Moore D, Smith JA, Seidman JG, Struhl K (1987) Current protocols in molecular biology. Wiley, New York
Bauernfeind JC (1981) Carotenoids as colorants and vitamin A precursors: technical and nutritional applications. Academic, New York
Ben-Amotz A, Avron M (1990) The biotechnology of cultivating the halotolerant alga Dunaliella. Trends Biotechnol 8:121–125
Boonyaratpalin M, Thongrod S, Supamattaya K, Britton G, Schlipalius LE (2001) Effects of β-carotene source, Dunaliella salina, and astaxanthin on pigmentation, growth, survival and health of Penaeus monodon. Aquac Res 32:182–190
Britton G, Liaaen-Jensen S, Pfander H (1998) Carotenoids. Birkhäuser Verlag, Basel
Cunningham FX Jr, Pogson B, Sun Z, McDonald KA, DellaPenna D, Gantt E (1996) Functional analysis of the beta and epsilon lycopene cyclase enzymes of Arabidopsis reveals a mechanism for control of cyclic carotenoid formation. Plant Cell 8:1613–1626
Dundas ID, Larsen H (1963) A study on the killing by light of photosensitized cells of Halobacterium salinarium. Arch Mikrobiol 46:19–28
Kovach ME, Elzer PH, Hill DS, Robertson GT, Farris MA, Roop RM 2nd, Peterson KM (1995) Four new derivatives of the broad-host-range cloning vector pBBR1MCS, carrying different antibiotic-resistance cassettes. Gene 166:175–176
Kuzina V, Cerda-Olmedo E (2006) Modification of sexual development and carotene production by acetate and other small carboxylic acids in Blakeslea trispora and Phycomyces blakesleeanus. Appl Environ Microbiol 72:4917–4922
Lampila LE, Wallen SE, Bullerman LB (1985) A review of factors affecting biosynthesis of carotenoids by the order Mucorales. Mycopathologia 90:65–80
Lee PC, Schmidt-Dannert C (2002) Metabolic engineering towards biotechnological production of carotenoids in microorganisms. Appl Microbiol Biotechnol 60:1–11
Lutnaes BF, Oren A, Liaaen-Jensen S (2002) New C40-carotenoid acyl glycoside as principal carotenoid in Salinibacter ruber, an extremely halophilic eubacterium. J Nat Prod 65:1340–1343
Nègre-Sadargues G, Castillo R, Segonzac M (2000) Carotenoid pigments and trophic behaviour of deep-sea shrimps (Crustacea, decapoda, alvinocarididae) from a hydrothermal area of the mid-atlantic ridge. Comp Biochem Physiol Part A Mol Integr Physiol 127:293–300
Nieto JJ, Fernandez-Castillo R, Marquez MC, Ventosa A, Quesada E, Ruiz-Berraquero F (1989) Survey of metal tolerance in moderately halophilic eubacteria. Appl Environ Microbiol 55:2385–2390
Ourisson G, Rohmer M, Poralla K (1987) Prokaryotic hopanoids and other polyterpenoid sterol surrogates. Annu Rev Microbiol 41:301–333
Rodríguez-Sáiz M, Paz B, de la Fuente JL, López-Nieto MJ, Cabri W, Barredo JL (2004) Genes for carotene biosynthesis from Blakeslea trispora. Appl Environ Microbiol 70:5589–5594
Sambrook J, Fritsch EF, Maniatis T (1989) Molecular cloning: a laboratory manual, 2nd edn. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, New York
Sandmann G (2003) Novel carotenoids genetically engineered in a heterologous host. Chem Biol 10:478–479
Straub O (1987) Key to carotenoids, 2nd edn. Birkhaüser, Basel
Sun Z, Gantt E, Cunningham FX Jr (1996) Cloning and functional analysis of the beta-carotene hydroxylase of Arabidopsis thaliana. J Biol Chem 271:24349–24352
Ulrich M (2000) Business report: the global market for carotenoids. Business Communication, Norwalk
Umeno D, Tobias AV, Arnold FH (2005) Diversifying carotenoid biosynthetic pathways by directed evolution. Microbiol Mol Biol Rev 69:51–78
Vargas C, Coronado MJ, Ventosa A, Nieto JJ (1997) Host range, stability, and compatibility of broad host-range-plasmids and a shuttle vector in moderately halophilic bacteria. Evidence of intragenic and intergenic conjugation in moderate halophiles. Syst Appl Microbiol 20:173–181
Ventosa A, Nieto JJ, Oren A (1998) Biology of moderately halophilic aerobic bacteria. Microbiol Mol Biol Rev 62:504–544
Vreeland RH, Litchfield CD, Martin EL, Elliot E (1980) Halomonas elongata, a new genus and species of extremely salt-tolerant bacteria. Int J Syst Bacteriol 30:485–495
Acknowledgment
We thank Dr. F. X. Cunningham for β-carotene biosynthetic genes and M. Sandoval, P. Merino, A. Morán, C. Flórez, and C. Aller for technical assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rodríguez-Sáiz, M., Sánchez-Porro, C., De La Fuente, J.L. et al. Engineering the halophilic bacterium Halomonas elongata to produce β-carotene. Appl Microbiol Biotechnol 77, 637–643 (2007). https://doi.org/10.1007/s00253-007-1195-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-007-1195-2