Abstract
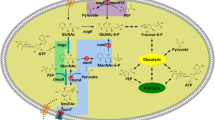
Whole-cell conversion of cyclohexanone to ɛ-caprolactone was attempted by recombinant Escherichia coli BL21(DE3) expressing cyclohexanone monooxygenase (CHMO) of Acinetobacter calcoaceticus NCIMB 9871. High concentrations of cyclohexanone and ɛ-caprolactone reduced CHMO-mediated bioconversion of cyclohexanone to ɛ-caprolactone in the resting recombinant E. coli cells. Metabolically active cells were employed by adopting a fed-batch culture to improve the production of ɛ-caprolactone from cyclohexanone. A glucose-limited fed-batch Baeyer–Villiger oxidation where a cyclohexanone level was maintained less than 6 g/l resulted in a maximum ɛ-caprolactone concentration of 11.0 g/l. The maximum ɛ-caprolactone concentration was improved further to 15.3 g/l by coexpression of glucose-6-phosphate dehydrogenase, an NADPH-generating enzyme encoded by the zwf gene which corresponded to a 39% enhancement in ɛ-caprolactone concentration compared with the control experiment performed under the same conditions.




Similar content being viewed by others
References
Alphand V, Carrea G, Wohlgemuth R, Furstoss R, Woodley JM (2003) Towards large-scale synthetic applications of Baeyer–Villiger monooxygenases. Trends Biotechnol 21:318–323
Baldwin CV, Woodley JM (2006) On oxygen limitation in a whole cellbiocatalytic Baeyer–Villiger oxidation process. Biotechnol Bioeng 95:362–369
Cheesman MJ, Kneller MB, Kelly EJ, Thompson SJ, Yeung CK, Eaton DL, Rettie AE (2001) Purification and characterization of hexahistidine-tagged cyclohexanone monooxygenase expressed in Saccharomyces cerevisiae and Escherichia coli. Protein Expr Purif 21:81–86
Doig SD, O’Sullivan LM, Patel S, Ward JM, Woodley JM (2001) Large scale production of cyclohexanone monooxygenase from Escherichia coli TOP10 pQR239. Enzyme Microb Technol 28:265–274
Doig SD, Avenell PJ, Bird PA, Gallati P, Lander KS, Lye GJ, Wohlgemuth R, Woodley JM (2002) Reactor operation and scale-up of whole cell Baeyer–Villiger catalyzed lactone synthesis. Biotechnol Prog 18:1039–1046
Doig SD, Simpson H, Alphand V, Furstoss R, Woodley J (2003) Characterization of a recombinant Escherichia coli TOP10 pQR239 whole-cell biocatalyst for stereoselective Baeyer–Villiger oxidations. Enzyme Microb Technol 32:347–355
Drepper T, Eggert T, Hummel W, Leggewie C, Pohl M, Rosenau F, Wilhelm S, Jaeger KE (2006) Novel biocatalysts for white biotechnology. Biotechnol J 1:777–786
Duetz WA, van Beilen JB, Witholt B (2001) Using proteins in their natural environment: potential and limitations of microbial whole-cell hydroxylations in applied biocatalysis. Curr Opin Biotechnol 12:419–425
Endo T, Koizumi S (2001) Microbial conversion with cofactor regeneration using genetically engineered bacteria. Adv Synth Catal 343:521–526
Hilker I, Baldwin C, Alphand V, Furstoss R, Woodley J, Wohlgemuth R (2006) On the influence of oxygen and cell concentration in an SFPR whole cell biocatalytic Baeyer–Villiger oxidation process. Biotechnol Bioeng 93:1138–1144
Hollmann F, Hofstetter K, Schmid A (2006) Non-enzymatic regeneration of nicotinamide and flavin cofactors for monooxygenase catalysis. Trends Biotechnol 24:163–171
Isken S, de Bont JAM (1998) Bacteria tolerant to organic solvents. Extremophiles 2:229–238
Kim JE, Kim EJ, Rhee WJ, Park TH (2005) Enhanced production of recombinant protein in Escherichia coli using silkworm hemolymph. Biotechnol Bioprocess Eng 1:353–356
Lee WH, Park YC, Lee DH, Park KM, Seo JH (2005) Simultaneous biocatalyst production and Baeyer–Villiger oxidation for bioconversion of cyclohexanone by recombinant Escherichia coli expressing cyclohexanone monooxygenase. Appl Biochem Biotechnol 121–124:827–836
Li Z, van Beilen JB, Duetz WA, Schmid A, de Raadt A, Griengl H, Witholt B (2002) Oxidative biotransformations using oxygenases. Curr Opin Chem Biol 6:136–144
Lim SJ, Jung YM, Shin HD, Lee YH (2002) Amplification of the NADPH-related genes zwf and gnd for the oddball biosynthesis of PHB in an E. coli transformant harboring a cloned phbCAB operon. J Biosci Bioeng 93:543–549
Mihovilovic MD, Rudroff F, Winninger A, Schneider T, Schulz F, Reetz MT (2006) Microbial Baeyer–Villiger oxidation: stereopreference and substrate acceptance of cyclohexanone monooxygenase mutants prepared by directed evolution. Org Lett 8:1221–1224
Na KI, Kim MD, Min WK, Kim JA, Lee WJ, Kim DO, Park KM, Seo JH (2005) Expression and purification of ubiquitin-specific protease (UBP1) of Saccharomyces cerevisiae in recombinant Escherichia coli. Biotechnol Bioprocess Eng 10:599–602
Panke S, Held M, Wubbolts MG, Witholt B, Schmid A (2002) Pilot-scale production of (S)-styrene oxide from styrene by recombinant Escherichia coli synthesizing styrene monooxygenase. Biotechnol Bioeng 80:33–41
Ryerson CC, Ballou DP, Walsh CT (1982) Mechanistic studies on cyclohexanone oxygenase. Biochemistry 21:2644–2655
Sambrook J, Russell RW (2001) Molecular cloning: a laboratory manual, 3rd edn. Cold Spring Harbor Laboratory Press, Cold Spring, New York
Sauer U, Canonaco F, Heri S, Perrenoud A, Fischer E (2004) The soluble and membrane-bound transhydrogenase udhA and pntB have divergent functions in NADPH metabolism of Escherichia coli. J Biol Chem 279:6613–6619
Stewart JD (1998) Cyclohexanone monooxygenase: a useful reagent for asymmetric Baeyer–Villiger reactions. Curr Org Chem 2:195–216
Tishkov VI, Gallkin AG, Fedorchuk VV, Savitsky PA, Rojkova AM, Gieren H, Kula MR (1999) Pilot scale production and isolation of recombinant NAD+- and NADP+-specific formate dehydrogenase. Biotechnol Bioeng 64:187–193
van der Donk W, Zhao H (2003) Recent developments in pyridine nucleotide regeneration. Curr Opin Biotechnol 14:421–426
Walton AZ, Stewart JD (2002) An efficient enzymatic Baeyer–Villiger oxidation by engineered Escherichia coli cells under non-growing conditions. Biotechnol Prog 18:262–268
Walton AZ, Stewart JD (2004) Understanding and improving NADPH-dependent reactions by nongrowing Escherichia coli cells. Biotechnol Prog 20:403–411
Zambianchi F, Pasta P, Carrea G, Colonna S, Gaggero N, Woodley JM (2002) Use of isolated cyclohexanone monooxygenase from recombinant Escherichia coli as a biocatalyst for Baeyer–Villiger and sulfide oxidations. Biotechnol Bioeng 78:489–496
Zhao J, Baba T, Mori H, Shimizu K (2004) Global metabolic response of Escherichia coli to gnd and zwf-knockout, based on 13C-labelling experiments and the measurements of enzyme activities. Appl Microbiol Biotechnol 64:91–98
Acknowledgments
This work was supported by the Korea Energy Management Corporation. W.H. Lee was supported by the Korea Ministry of Education through the BK21 program. M.D. Kim was supported in part by MAF/ARPC through Grape Research Project Group.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Lee, WH., Park, JB., Park, K. et al. Enhanced production of ɛ-caprolactone by overexpression of NADPH-regenerating glucose 6-phosphate dehydrogenase in recombinant Escherichia coli harboring cyclohexanone monooxygenase gene. Appl Microbiol Biotechnol 76, 329–338 (2007). https://doi.org/10.1007/s00253-007-1016-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-007-1016-7