Abstract
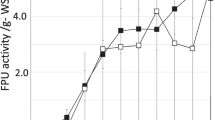
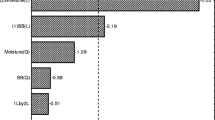
To improve efficiency and reduce cost, solid state simultaneous saccharification and fermentation of peashrub woody biomass was investigated under anaerobic conditions at 50°C, with a cellulase–inoculant mixture consisting of Trichoderma koningii cellulase, Aspergillus niger cellulase, and Lactobacillus. Experimental formulations were prepared according to uniform prescription design principles. By crude protein, crude fiber models constructed using multivariate regression in SPSS and solutions analysis through unconstrained mathematical optimization in Microsoft Excel, it was clearly revealed that low pH value (3.8) from lactic acid accumulation produced by Lactobacillus would ultimately limit enzymatic hydrolysis during long-term fermentation (30 days). It was shown that a cellulase complex with filter paper cellulase/carboxymethyl cellulase/cotton lyase/β-glucosidase/pectinase of activity ratios of 0.6:1:0.3:1:2.6 could effectively break peashrub cell wall structure by biodegradation of easily digested components and, then, release cellular contents to improve crude protein content. Thus, the enzymatic hydrolysis of peashrub biomass by the optimized cellulase complex could improve crude protein content by 45.3% (from 8.45 to 12.28%), although it only biodegraded about 10.90% of the crude fiber (from 44.45 to 40.08%).




Similar content being viewed by others
References
Bustos G, Moldes AB, Cruz JM, Domínguez JM (2004) Production of lactic acid from vine-trimming wastes and viticulture lees using a simultaneous saccharification fermentation method. J Sci Food Agric 85(3):466–472
Fang KT, Ma CX (2001) Orthogonal and uniform experimental design. Sciences Press, Beijing, pp 144–152
Grabber JH, Hatfield RD, Ralph J (1999) Diferulate cross-links impede the enzymatic degradation of non-lignified maize walls. J Sci Food Agric 77(2):193–200
Hatfield RD (1993) Cell wall polysaccharide interactions and degradability. In: Jung HG, Buxton DR, Hatifeld RD, Ralph J (eds) Forage cell wall structure and digestibility. American Society of Agronomy, Madison, WI, USA, pp 285–313
Jaakkola S, Huhtanen P, Hissa K (1991) The effect of cell wall degrading enzymes or formic acid on fermentation quality and on digestion of grass silage by cattle. Grass Forage Sci 46:75–87
Jing DB (2004) The utilization channel and key technology of peashrub in Ordos area. Dissertation for Ph.D. Graduate school, Chinese Academy of Sciences, Shenyang, PR China, pp 82–114
Jing DB, Li PJ, Frank S, Xiong XZ, Ling L (2006) Pectinase production by solid fermentation from Aspergillusniger by a new prescription experiment. Ecotoxicol Environ Saf 64:244–250
Kabel MA, van der Maarel MJ, Klip G, Voragen AG, Schols HA (2006) Standard assays do not predict the efficiency of commercial cellulase preparations towards plant materials. Biotechnol Bioeng 93(1):56–63
Koukiekolo R, Cho HY, Kosugi A, Inui M, Yukawa H, Doi RH (2005) Degradation of corn fiber by Clostridium cellulovorans cellulases and hemicellulases and contribution of scaffolding protein CbpA. Appl Environ Microbiol 71(7):3504–3511
Kung JL, Tung RS, Maciorowski KG, Buffum K, Knutsen K, Aimutis WR (1991) Effects of plant cell-wall-degrading enzymes and lactic acid bacteria on silage fermentation and composition. J Dairy Sci 74:4284–4296
Li PJ, Jing DB, Zhou QX, Zhang CG (2004) Optimization of solid fermentation of cellulase from Trichoderma koningii. J Environ Sci (China) 5:816–820
Lin Y, Tanaka S (2006) Ethanol fermentation from biomass resources: current state and prospects. Appl Microbiol Biotechnol 69(6):627–642
Lynd LR, Cushman JH, Nichols RJ, Wyman CE (1991) Fuel ethanol from cellulosic biomass. Science 251:1318–1323
Murashima K, Kosugi A, Doi RH (2003) Synergistic effects of cellulosomal xylanase and cellulases from Clostridium cellulovorans on plant cell wall degradation. J Bacteriol 185(5):1518–1524
Nadeau EMG, Buxton DR (1997) Cellulase and bacterial inoculant effects on cocksfoot and lucerne ensiled at high dry matter levels. J Sci Food Agric 73:369–376
Patel MA, Ou MS, Ingram LO, Shanmugam KT (2005) Simultaneous saccharification and co-fermentation of crystalline cellulose and sugar cane bagasse hemicellulose hydrolysate to lactate by a thermotolerant acidophilic Bacillus sp. Biotechnol Prog 21(5):1453–1460
Solaiman DKY, Ashby RD, Foglia TA, Marmer WN (2006) Conversion of agricultural feedstock and coproducts into poly(hydroxyalkanoates). Appl Microbiol Biotechnol 71(6):783–789
Stokes MR (1992) Effects of an enzyme mixture, an inoculant and their interaction on silage fermentation and dairy production. J Dairy Sci 75:764–773
van WyK JPH (2001) Sequential bioconversion of used paper to sugars by cellulases from Trichoderma reesei and Penicillium funiculosum. Environmentalist 21:211–220
Walker LP, David BW (1997) Optimizing of cellulase mixtures for maximum rate and extent of hydrolysis: final report. New York State Energy Research and Development Authority, 1150-ERER-ER-89
Wang L, Zhang Y, Gao P, Shi D, Liu H, Gao H (2006) Changes in the structural properties and rate of hydrolysis of cotton fibers during extended enzymatic hydrolysis. Biotechnol Bioeng 93(3):443–456
Wood BE, Aldrich HC, Ingram LO (1997) Ultrasound stimulates ethanol production during the simultaneous saccharification and fermentation of mixed waste office paper. Biotechnol Prog 13(3):232–237
Yang XX, Chen HZ, Li ZH (2001) Bioconversion of corn straw by coupling ensiling and solid state fermentation. Bioresour Technol 78:277–280
Yu Z, Naoki Ni, Yoshiro K, Senji U (1999) Ensiling characteristics and ruminal degradation of Italian ryegrass and lucerne silages treated with cellwall-degrading enzymes. J Sci Food Agric 79:1987–1992
Zhang DQ, Zhang ZY, Huang ZY (2000) Conversion of ligno-cellulosic biomass to ethanol by the SSF process (III). J Beijing For Univ (in Chinese) 6:50–54
Zhu QS, Yan LF, Guo QX (2002) Clean energy from biomass. Chemical Industry Press, Beijing, pp 46–47
Acknowledgments
The authors are grateful to Professor Tom McRae, Vice Professor Chang Shijun, Zhang Chungui, Xu Huaxia, and other participants in the Analysis and Test Center of Shenyang Agricultural University for their assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jing, D., Li, P., Xiong, XZ. et al. Optimization of cellulase complex formulation for peashrub biomass hydrolysis. Appl Microbiol Biotechnol 75, 793–800 (2007). https://doi.org/10.1007/s00253-007-0891-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-007-0891-2