Abstract
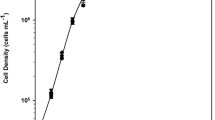
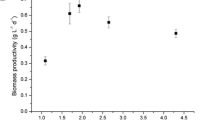
The underwater tubular photobioreactor is a fully controlled outdoor system to study photosynthetic bacteria. Before growing bacteria cells outdoors, two modified van Niel medium (vN-A, vN-B) were tested under artificial light. During exponential growth, the specific growth rates were 0.0416 and 0.0434 h−1, respectively; vN-B was chosen for outdoor experiments. The growth behavior of Rhodopseudomonaspalustris was investigated under a natural light–dark cycle (sunrise–sunset, 15L/9D) and a forced light–dark cycle (9:00–19:00, 10L/14D). Relationships between solar radiations, daily growth rates, and biomass output rates were also investigated. After determining the elemental biomass molar composition and its combustion heat, some trends of photosynthetic efficiency (PE) were obtained over daylight. The PE trends were always of the oscillatory type, with the exception of that achieved at low biomass concentration. Under a natural light/dark cycle, the maximum PE (11.2%) was attained at sunset, while under a forced light/dark cycle, the highest PE (8.5%) was achieved in the morning. Three initial biomass concentrations were investigated (0.65, 1.01, and 1.54 g l−1). The stoichiometric equation for bacteria cells indicated that 87.7% of the carbon of acetic acid was converted to biomass and only 12.3% was lost as CO2.






Similar content being viewed by others
References
Adir N, Zer H, Shochat S, Ohad I (2003) Photoinhibition—a historical perspective. Photosynth Res 76:343–370
Andersson B, Aro EM (1997) Proteolytic activities and proteases of plant chloroplasts. Physiol Plant 100:780–793
Barber J (1991) Photoinactivation of the isolated Photosystem II reaction center and its prevention. In: Douglas RH, Moan J, Ronto G (eds) Light biology and medicine. Plenum, New York, pp 21–22
Barber J, Andersson B (1992) Too much of a good thing: light can be good and bad for photosynthesis. Trends Biochem Sci 17:61–66
Barbosa MR, Rocha JMS, Tramper J, Wijffels RH (2001) Acetate as carbon source for hydrogen production by photosynthetic bacteria. J Biotechnol 85:25–33
Behrenfeld MJ, Prasil O, Kolber ZS, Babin M, Falkowski PG (1998) Compensatory changes in Photosystem II electron turnover rates protect photosynthesis from photoinhibition. Photosynth Res 58:259–268
Burnap RL (2004) D1 protein processing and Mn cluster assembly in light of the emerging Photosystem II structure. Phys Chem Chem Phys 6:4803–4809
Carlozzi P, Sacchi A (2001) Biomass production and studies on Rhodopseudomonas palustris grown in an outdoor, temperature controlled, underwater tubular photobioreactor. J Biotechnol 88:239–249
D’Adamo PD, Rozich AF, Gaudy AF Jr (1984) Analysis of growth data with inhibitory carbon sources. Biotechnol Bioeng 26:397–402
England PG, Gibson J, Harwood CS (1991) Reductive coenzyme A-mediated pathway for 3-chlorobenzenzoate degradation in the phototrophic bacterium Rhodopseudomonas palustris. Appl Environ Microbiol 67(3):1396–1399
Eroğlu E, Gündüz U, Yücel M, Türker L, Eroğlu I (2004) Photobiological hydrogen production by using olive mill wastewater as a sole substrate source. Int J Hydrogen Energy 29:163–171
Evans MB, Hawthornthwaite AM, Cogdell RJ (1990) Isolation and characterisation of the different B800–850 light-harvesting complexes from low and high-light grown cells of Rhodopseudomonas palustris, strain 2.1.6. Biochim Biophys Acta 1016:71–76
Fißler J, Kohring GW, Giffhorn F (1995) Enhanced hydrogen production from aromatic acids by immobilized cells of Rhodopseudomonas palustris. Appl Microbiol Biotechnol 44:43–46
Gibson KJ, Gibson J (1992) Potential early intermediates in anaerobic benzoate degradation by Rhodopseudomonas palustris. Appl Environ Microbiol 2:696–698
Goldman JC (1979) Outdoor algal mass cultures, II. Photosynthetic yield limitations. Water Res 13:119–136
Hirotani H, Ohigashi H, Kobayashi M, Koshimizu K, Takahashi E (1991) Inactivation of T5 phage by cis-vaccenic acid, an antivirus substance from Rhodopseudomonas capsulata, and unsaturated fatty acids and related alcohols. FEMS Microbiol Lett 77:13–18
Imai Y, Morita S, Arata Y (1984) Proton correlation NMR studies of metabolism in Rhodopseudomonas palustris. J Biochem 96:691–699
Kenyon CN (1978) Complex lipids and fatty acids of photosynthetic bacteria. In: Clayton RK, Sistrom WR (eds) The photosynthetic bacteria. Plenum, New York, pp 281–313
Kyle DJ (1978) The biochemical basis of photoinhibition of Photosystem II. In: Kyle DJ, Osmond CB, Arntzen CJ (eds) Photoinhibition. Elsevier, Amsterdam, pp 197–226
Melis A (1999) Photosystem-II damage and repair cycle in chloroplasts: what modulates the rate of photodamage in vivo? Trends Plant Sci 4:130–135
Monod J (1949) The growth of bacterial cultures. Annu Rev Microbiol 3:371–394
Pfennig N (1978) General physiology and ecology of photosynthetic bacteria. In: Clayton RK, Sistrom WR (eds) The photosynthetic bacteria. Plenum, New York, pp 3–18
Prasil O, Adir N, Ohad I (1992) Dynamics of Photosystem II: mechanism of photoinhibition and recovery processes. In: Barber J (ed) The photosystems: structure, function and molecular biology. Elsevier, Amsterdam, pp 295–348
Rozich AF, Colvin RJ (1986) Effects of glucose on phenol biodegradation by heterogeneous populations. Biotechnol Bioeng 28:965–971
Segers L, Verstrynge L, Verstraete W (1981) Product patterns of non-axenic sucrose fermentation as a function of pH. Biotechnol Lett 3(11):635–640
Sheller HV, Haldrup A (2005) Photoinhibition of photosystem I. Planta 221:5–8
Spoehr HA, Milner HW (1949) The chemical composition of Chlorella; effect of environmental conditions. Plant Physiol 24:120–149
Sukenik A, Falkowski PG, Bennett J (1987) Potential enhancement of photosynthetic energy conversion in algal mass culture. Biotechnol Bioeng 30:970–977
Tandori J, Hideg E, Nagy L, Maroti P, Vass I (2001) Photoinhibition of carotenoidless reaction centers from Rhodobacter sphaeroides by visible light. Effects on protein structure and electron transport. Photosynth Res 70:175–184
Trüper HG, Pfenning N (1978) Taxonomy of the Rhodospirillales. In: Clayton RK, Sistrom WR (eds) The photosynthetic bacteria. Plenum, New York, pp 19–27
Vrati S (1984) Single cell protein production by photosynthetic bacteria grown on the clarified effluents of biogas plant. Appl Microbiol Biotechnol 19:199–202
Acknowledgements
The authors wish to thank Mr. A. Sacchi for his technical assistance and Mr. E. Pinzani for his instrumentation management of the photobioreactor.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Carlozzi, P., Pushparaj, B., Degl’Innocenti, A. et al. Growth characteristics of Rhodopseudomonas palustris cultured outdoors, in an underwater tubular photobioreactor, and investigation on photosynthetic efficiency. Appl Microbiol Biotechnol 73, 789–795 (2006). https://doi.org/10.1007/s00253-006-0550-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-006-0550-z