Abstract
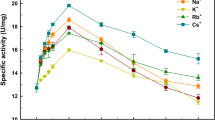
A truncated gene from Bacillus lichenifromis ATCC 27811 encoding a recombinant γ-glutamyltranspeptidase (BLrGGT) was cloned into pQE-30 to generate pQE-BLGGT, and the overexpressed enzyme was purified from the crude extract of IPTG-induced E. coli M15 (pQE-BLGGT) to homogeneity by nickel-chelate chromatography. This protocol yielded over 25 mg of purified BLrGGT per liter of growth culture under optimum conditions. The molecular masses of the subunits of the purified enzyme were determined to be 41 and 22 kDa, respectively, by sodium dodecyl sulfate-polyacrylamide gel electrophoresis. The optimum pH and temperature for the recombinant enzyme were 6–8 and 40 °C, respectively. The chloride salt of metal ions Mg2+, K+, and Na+ can activate BLrGGT, whereas that of Pb2+ dramatically inhibited it. The substrate specificity study showed that l-γ-glutamyl-p-nitroanilide (l-γ-Glu-p-NA) is a preference for the enzyme. Steady-state kinetic study revealed that BLrGGT has a k cat of 105 s−1 and a K m of 21 μM when using l-γ-Glu-p-NA as the substrate. With this overexpression and purification system, BLrGGT can now be obtained in quantities necessary for structural characterization and synthesis of commercially important γ-glutamyl compounds.





Similar content being viewed by others
References
Brannigan JA, Dodson G, Duggelby HJ, Moody PC, Smith JL, Tomchick DR, Murzin AG (1995) A protein catalytic framework with an N-terminal nucleophile is capable of self-activation. Nature (London) 378:416–419
Carrio MM, Villaverade A (2001) Protein aggregation as bacterial inclusion bodies is reversible. FEBS Lett 489:29–33
Chevalier C, Thiberge JM, Ferrero RL, Labigne A (1999) Essential role of Helicobacter pylori γ-glutamyltranspeptidase for the colonization of the gastric mucosa of mice. Mol Microbiol 31:1359–1372
Choi JH, Lee SY (2004) Secretory and extracellular production of recombinant proteins using Escherichia coli. Appl Microbiol Biotechnol 64: 625–635
Coloma J, Pitot HC (1986) Characterization and sequence of cDNA clone of γ-glutamyltranspeptidase. Nucleic Acids Res 14:1393–1403
Ditzel L, Huber R, Mann K, Heinemeyer W, Wolf DH, Groll M (1998) Conformational constraints for protein self-cleavage in the proteasome. J Mol Biol 279:1187–1191
Doi RH, Rodriguez RL, Trait RC (1983) Recombinant DNA techniques: an introduction. Addison-Wesley, MA, USA, pp 162–164
Eder J, Fersht AR (1995) Pro-sequence-assisted protein folding. Mol Microbiol 16: 609–614
Feller G, D’Amico S, Benotmane AM, Joly F, Beeumen JV, Genday C (1998) Characterization of the C-terminal propeptide involved in bacterial wall spanning of α-amylase from the psychrophile Alteromonas haloplanctis. J Biol Chem 273: 12109–12115
Goodspeed D, Dunn T, Miller C, Pitot H (1989) Human γ-glutamyl transpeptidase cDNA: comparison of hepatoma and kidney mRNA in the human and rat. Gene 76:1–9
Guan C, Cui T, Rao V, Liao W, Benner J, Lin CL, Comb D (1996) Activation of glycosylasparaginase: formation of active N-terminal threonine by intramolecular autoproteolysis. J Biol Chem 271:1732–1737
Guan C, Liu Y, Shao Y, Cui T, Liao W, Whitaker R, Paulus H (1998) Characterization and functional analysis of the cis-autoproteolysis active center of glycosylasparaginase. J Biol Chem 273:20205–20212
Hashimoto W, Suzuki H, Yamamoto K, Kumagai H (1995) Effect of site-directed mutations on processing and activity of γ-glutamyltranspeptidase of Escherichia coli K-12. J Biochem (Tokyo) 118:75–80
Hill DW, Walters FH, Wilson TD, Stuart JD (1979) High performance liquid chromatographic determination of amino acids in the picomole range. Anal Chem 51:1338–1341
Hockney RC (1994) Recent developments in heterologous protein production in Escherichia coli. Trends Biotechnol 12:456–463
Hodgson J (1993) Expression systems: a user’s guide. Bio/Technology 11:887–893
Ikeda Y, Fuiji J, Taniguchi N (1993) Significance of Arg-107 and Glu-108 in the catalytic mechanism of human γ-glutamyl transpeptidase: identification by site-directed mutagenesis. J Biol Chem 268:3980–3986
Ikeda Y, Fuiji J, Anderson ME, Taniguchi N, Meister A (1995) Involvement of Ser-451 and Ser-452 in the catalysis of human γ-glutamyl transpeptidase. J Biol Chem 270:22223–22228
Ikeda Y, Fuiji J, Taniguchi N (1996) Effects of substitutions of the conserved histidine residues in human γ-glutamyl transpeptidase. J Biochem 119:1166–1170
Inoue M, Hiratake J, Suzuki H, Kumagai H, Sakata K (2000) Identification of catalytic nucleophile of Escherichia coli γ-glutamyltranspeptidase by γ-monofluophosphono derivative of glutamic acid: N-terminal Thr-391 in the small subunit is the nucleophile. Biochemistry 39:7764–7771
Ishiye M, Yamashita M, Niwa M (1993) Molecular cloning of the γ-glutamyltranspeptidase gene from a Pseudomonas strain. Biotechnol Prog 9:323–331
Izard JW, Kendall DA (1994) Signal peptides: exquisitely designed transport promoters. Mol Microbiol 13: 765–773
Jones AW, Smith DA, Watkins JC (1984) Structure-activity relations of dipeptide antagonists of excitatory amino acids. Neuroscience 13:573–581
Kiefhaber T, Rudolph R, Kohler HH, Buchner J (1991) Protein aggregation in vitro and in vivo: a quanitative model of the kinetic competition between folding and aggregation. Bio/Technology 9:211–218
Kim Y, Kim S, Earnest TN, Hol WG (2002) Precursor structure of cephalosporin acylase: insights into autoproteolytic activation in a new N-terminal hydrolase family. J Biol Chem 277:2823–2829
Kim JK, Yang IS, Rhee S, Dauter Z, Lee YS, Park SS, Kim KH (2003) Crystal structures of glutaryl 7-aminocephalocephalosporanic acid acylase: insight into autoproteolytic activation. Biochemistry 42:4084–4093
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature (London) 227:680–685
Lammertyn E, Anné J (1998) Modification of Streptomyces signal peptides and their effects on protein production and secretion. FEMS Microbiol Lett 160: 1–10
Laperche Y, Bulle F, Aissani TM, Aggerbeck M, Hanoune J, Guellaen G (1986) Molecular cloning of rat kidney γ-glutamyltranspeptidase cDNA. Proc Natl Acad Sci USA 83:937–941
Marchesini G, Avagnina S, Barantani EG, Ciccarone AM, Corcia F, Dall’aglio E, Dalle Grave R, Morpurgo PS, Tomasi F, Vitacolonna E (2005) Aminotransferase and γ-glutamyltranspeptidase levels in obesity are associated with insulin resistance and the metabolic syndrome. J Endocrinol Invest 28:333–339
Meister A (1973) On the enzymology of amino acid transport. Science 180:33–39
Meister A, Anderson ME (1983) Glutathione. Annu Rev Biochem 52:711–760
Meister A, Tate S (1985) γ-Glutamyl transpeptidase from kidney. Methods Enzymol 113:400–419
Nakayama R, Kumagai H, Tuchikura T (1984) Purification and properties of γ-glutamyltranspeptidase from Proteus mirabilis. J Bacteriol 160:341–346
Ogawa Y, Hosoyama H, Hamano M, Motai H (1991) Purification and properties of γ-glutamyltranspeptidase from Bacillus subtilis (natto). Agric Biol Chem 55:2971–2977
Oinonen C, Rouvinen J (2000) Structural comparison of Ntn-hydrolases. Protein Sci 9:2329–2337
Olins PO, Lee SC (1993) Recent advances in heterologous gene expression in Escherichia coli. Curr Opin Biotechnol 4:520–525
Orlowski M, Meister A (1963) γ-Glutmyl-p-nitroanilide: a new convenient substrate for determination and study of l- and d-γ-glutamyltranspeptidase activities. Biochim Biophys Acta 73:679–681
Orlowski M, Meister A (1965) Isolation of γ-glutamyltranspeptidase from hog kidney. J Biol Chem 240: 338–347
Orlowski M, Meister A (1970) The γ-glutamyl cycle: a possible transport system for amino acids. Proc Natl Acad Sci USA 67:1248–1255
Rey MW, Ramaiya P, Nelson BA, Brody-Karpin SD, Zaretsky EJ, Tang M, Lopez de Leon A, Xiang H, Gusti V, Clausen IG, Olsen PB, Rasmussen MD, Andersen JT, Jørgensen PL, Larsen TS, Sorokin A, Bolotin A, Lapidus A, Galleron N, Ehrlich SD, Randy MB (2004) Complete genome sequence of the industrial bacterium Bacillus licheniformis and comparison with closely related Bacillus species. Genome Biol 5:R77
Robins R, Davies D (1981) The role of glutathione in amino-acid absorption. Biochem J 194:63–70
Sambrook J, Russell DW (2001) Molecular cloning: a laboratory manual, 3rd edn. Cold Spring Harbor Laboratory, Cold Spring Harbor, NY, USA
Schein CH (1989) Production of soluble recombinant proteins in bacteria. Bio/Technology 7:1141–1148
Simbirtsey A, Kolobov A, Zabolotnych N, Pigareva N, Konusova V, Kotov A, Variouchina E, Bokovanov V, Vinogradova T, Vasilieva S, Tuthill C (2003) Biological activity of peptide SCV-07 against murine tuberculosis. Russ J Immunol 8:11–22
Smith TK, Meister A (1995) Chemical modification of active site residues in γ-glutamyltranspeptidase: aspartate 422 and cysteine 453. J Biol Chem 270:12476–12480
Smith TK, Ikeda Y, Fuiji J, Taniguchi N, Meister A (1995) Different sites of acivicin binding and inactivation of γ-glutamyltranspeptidases. Proc Natl Acad Sci USA 92:2360–2364
Sørensen HP, Mortensen KK (2005) Advanced genetic strategies for recombinant protein expression in Escherichia coli. J Biotechnol 115:113–128
Stole E, Seddon AP, Welner D, Meister A (1990) Identification of a highly reastive threonine residue at the active site of γ-glutamyltranspeptidase. Proc Natl Acad Sci USA 87:1706–1709
Suzuki H, Kumagai H, Tochikura T (1986) γ-Glutamyltranspeptidase from Escherichia coli K-12: purification and properties. J Bacteriol 168: 1325–1331
Suzuki H, Kumagai H, Echigo T, Tochikura T (1989) DNA sequence of the Escherichia coli K-12 γ-glutamyltranspeptidase gene ggt. J Bacteriol 171:5169–5172
Suzuki H, Hashimoto W, Kumagai H (1993) Escherichia coli K-12 can utilize an exogenous γ-glutamyl peptide as a amino source, for which γ-glutamyltranspeptidase is essential. J Bacteriol 175:6038–6040
Suzuki H, Izuka S, Minami H, Miyakawa N, Ishihara S, Kumagai H (2003) Use of bacterial γ-glutamyltranspeptidase for enzymatic synthesis of γ-d-glutamyl compounds. Appl Environ Microbiol 69:6399–6404
Suzuki H, Kato K, Kumagai H (2004) Development of an efficient enzymatic production of γ-d-glutamyl-l-tryptophan (SCV-07), a prospective medicine for tuberculosis, with bacterial γ-glutamyltranspeptidase. J Biotechnol 111:291–295
Tate S, Meister A (1981) γ-Glutamyl transpeptidase: catalytic, structural and function aspects. Mol Cell Biochem 39:357–368
Xu K, Strauch MA (1996) Identification, sequence, and expression of the gene encoding γ-glutamyltranspeptidase in Bacillus subtilis. J Bacteriol 178:4319–4322
Xu Q, Buckley D, Guan C, Guo HC (1999) Structural insights into the mechanism of intramolecular proteolysis. Cell 98:651–661
Acknowledgements
We are grateful to Dr. Kuo-Lung Ku for technical assistance in some of the analytical experiments, as well as Dr. Wenlung Chen for the facility support during the purification of the recombinant enzyme. This work was supported in part by a grant (NSC 94-2313-B-415-002) from National Science Council of Taiwan, Republic of China.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lin, LL., Chou, PR., Hua, YW. et al. Overexpression, one-step purification, and biochemical characterization of a recombinant γ-glutamyltranspeptidase from Bacillus licheniformis . Appl Microbiol Biotechnol 73, 103–112 (2006). https://doi.org/10.1007/s00253-006-0440-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-006-0440-4