Abstract
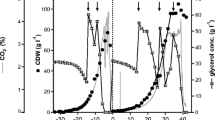
The current investigation focuses on shedding further light on the characteristics of lipase A from Candida antarctica (CalA), which has attracted growing attention in its suitability for industrial applications. CalA was functionally expressed in the methylotrophic yeast Pichia pastoris, purified and characterised. A classical fed-batch process and a semi-continuous process were developed and tested with regard to their yield capacity. Lipase concentrations of 0.88 and 0.55 g l−1 were obtained using the fed-batch and semi-continuous processes, respectively. The semi-continuous process reaches a total activity of 10,233,000 U and so surpasses the fed-batch process reaching 7,530,000 U. The purified enzyme showed highest activity between 50 and 70 °C at pH 7.0 and a preference for short-chain triglycerides (C4-C8). Significantly reduced activity was observed in the presence of hydrophilic esters.





Similar content being viewed by others
References
Anderson EM, Larsson KM, Kirk O (1998) One biocatalyst—many applications: the use of Candida antarctica lipase B—lipase in organic synthesis. Biocatal Biotransform 16:181–204
Bateman A, Birney E, Durbin R, Eddy SR, Howe KL, Sonnhammer EL (2000) The Pfam protein families database. Nucleic Acids Res 28(1):263–266
Björkling F, Godtfredsen SE, Kirk O (1991) The future impact of industrial lipases. Trends Biotechnol 9:360–363
Blum H, Beier H, Gross HJ (1987) Improved silver-staining of plant proteins, RNA and DNA in polyacrylamid gels. Electrophoresis 8:93–99
Borgdorf R, Warwel S (1999) Substrate selectivity of various lipases in the esterification of cis- and trans-9-octadecenoic acid. Appl Microbiol Biotechnol 51(4):480–485
Bornscheuer UT, Pleiss J, Schmidt-Dannert C, Schmid RD (1998) Lipases from Rhizopus oryzae: genetics, structure and application. In: Alberghina L (ed) Protein engineering in industrial biotechnology. Harwood Academic, Amsterdam, pp 115–134
Bradford M (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Cregg JM, Cereghino JL, Shi J, Higgins DR (2000) Recombinant protein expression in Pichia pastoris. Mol Biotechnol 16(1):23–52
Daly R, Hearn MTW (2003) Expression of heterologous proteins in Pichia pastoris: a useful experimental tool in protein engineering and production. J Mol Recognit 18:119–138
Dominguez de Maria P, Carboni-Oerlemans C, Tuin B, Bargeman G, van der Meer A, van Gemert R (2005) Biotechnological applications of Candida antarctica lipase A: state-of-the-art. J Mol Catal B Enzym 37:36–46
Fukusaki E, Satoda S, Senda S, Omata T (1999) Lipase-catalyzed kinetic resolution of 2,3-epoxy-1-tridecanol and its application to facile synthesis of (+)-disparlure. J Biosci Bioeng 87(1):103–104
Heldt-Hansen HP, Ishii M, Patkar SA, Hansen TT, Eigtved P (1989) Biocatalysis in agricultural biotechnology. American Chemical Society, Washington, District of Columbia, p 158
Henke E, Pleiss J, Bornscheuer UT (2002) Activity of lipases and esterases towards tertiary alcohols: insights into structure-function relationships. Angew Chem Int Ed Engl 41(17):3211–3213
Henke E, Bornscheuer UT, Schmid RD, Pleiss J (2003) A molecular mechanism of enantiorecognition of tertiary alcohols by carboxylesterases. Chembiochem 4(6):485–493
Hoegh I, Patkar S, Halkier T, Hansen MT (1995) Two lipases from Candida antarctica: cloning and expression in Aspergillus oryzae. Can J Bot 73:869–875
Invitrogen (1997) EasySelect Pichia expression kit—a manual of methods for expression of recombinant proteins using pPICZ and pPICZalpha in Pichia pastoris
Jahic M, Rotticci-Mulder JC, Martinelle M, Hult K, Enfors SO (2002) Modelling of growth and energy metabolism of Pichia pastoris producing a fusion protein. Bioprocess Biosyst Eng 24:385–393
Jahic M, Wallberg F, Bollok M, Garcia P, Enfors SO (2003) Temperature limited fed-batch technique for control of proteolysis in Pichia pastoris bioreactor cultures. Microb Cell Fact 2(1):6
Kaieda M, Nagayoshi M, Hama S, Kondo A, Fukuda H (2004) Enantioselective transesterification using immobilized Aspergillus oryzae overexpressing lipase. Appl Microbiol Biotechnol 65(3):301–305
Kakugawa K, Shobayashi M, Suzuki O, Miyakawa T (2002a) Cloning, characterization, and expression of cDNA encoding a lipase from Kurtzmanomyces sp. I-11. Biosci Biotechnol Biochem 66(6):1328–1336
Kakugawa K, Shobayashi M, Suzuki O, Miyakawa T (2002b) Purification and characterization of a lipase from the glycolipid-producing yeast Kurtzmanomyces sp. I-11. Biosci Biotechnol Biochem 66(5):978–985
Kirk O, Christensen MW (2002) Lipases from Candida antarctica: unique Biocatalysts from a unique origin. Org Process Res Dev 6:446–451
Kojima Y, Yokoe M, Mase T (1994) Purification and characterization of an alkaline lipase from Pseudomonas fluorescens AK102. Biosci Biotechnol Biochem 58(9):1564–1568
Krishna H, Persson MM, Bornscheuer UT (2002) Enantioselective transesterification of a tertiary alcohol by Lipase A from Candida antarctica. Tetrahedron Asymmetry 13:2693–2696
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227(5259):680–685
Martinelle M, Holmquist M, Hult K (1995) On the interfacial activation of Candida antarctica lipase A and B as compared with Humicola lanuginosa lipase. Biochim Biophys Acta 1258(3):272–276
Matsumoto T, Ito M, Fukuda H, Kondo A (2004) Enantioselective transesterification using lipase-displaying yeast whole-cell biocatalyst. Appl Microbiol Biotechnol 64(4):481–485
Nakano H, Kitahata S, Tominaga Y, Takenishi S (1991) Esterification of glycosides with glycerol and trimethyloipropane moieties by Candida cylindracea lipase. Agric Biol Chem 55:2083–2089
Neugnot V, Moulin G, Dubreucq E, Bigey F (2002) The lipase/acyltransferase from Candida parapsilosis: molecular cloning and characterization of purified recombinant enzymes. Eur J Biochem 269(6):1734–1745
Ollis DL, Cheah E, Cygler M, Dijkstra B, Frolow F, Franken SM, Harel M, Remington SJ, Silman I, Schrag J (1992) The alpha/beta hydrolase fold. Protein Eng 5(3):197–211
Otto RT, Scheib H, Bornscheuer UT, Pleiss J, Syldatk C, Schmid RD (2000) Substrate specificity of lipase B from Candida antarctica in the synthesis of arylaliphatic glycolipids. J Mol Catal B Enzym 8:201–211
Rogalska E, Cudrey C, Ferrato F, Verger R (1993) Stereoselective hydrolysis of triglycerides by animal and microbial lipases. Chirality 5:24–30
Rusnak M, Nieveler J, Schmid RD, Petri R (2005) The putative lipase, AF1763, from Archaeoglobus fulgidus is a carboxylesterase with a very high pH optimum. Biotechnol Lett 27(11):743–748
Sahin N, Akoh CC, Karaali A (2005) Lipase-catalyzed acidolysis of tripalmitin with hazelnut oil fatty acids and stearic acid to produce human milk fat substitutes. J Agric Food Chem 53(14):5779–5783
Sakaki K, Itoh N (2003) Optical resolution of racemic 2-hydroxy octanoic acid by lipase-catalyzed hydrolysis in a biphasic membrane reactor. Biotechnol Lett 25(19):1591–1595
Sanger F, Nicklen S, Coulsen AR (1977) DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci USA 74:5463–5467
Schmid RD, Verger R (1998) Lipases, interfacial enzymes with attractive apllications. Agnew Chem Int Ed Engl 37:1608–1633
Schmidt M, Barbayianni E, Fotakopoulou I, Hohne M, Constantinou-Kokotou V, Bornscheuer UT, Kokotos G (2005) Enzymatic removal of carboxyl protecting groups. 1. Cleavage of the tert-butyl moiety. J Org Chem 70(9):3737–3740
Schmidt-Dannert C (1999) Recombinant microbial lipases for biotechnological applications. Bioorg Med Chem 7(10):2123–2130
Solymár M, Fülöp F, Kanerva LT (2002) Candida antarctica lipase A—a powerful catalyst for the resolution of heteroaromatic beta-amino esters. Tetrahedron Asymmetry 13:2383–2388
Tang SJ, Shaw JF, Sun KH, Sun GH, Chang TY, Lin CK, Lo YC, Lee GC (2001) Recombinant expression and characterization of the Candida rugosa lip4 lipase in Pichia pastoris: comparison of glycosylation, activity, and stability. Arch Biochem Biophys 387(1):93–98
Tsuchiya D, Murakami Y, Ogoma Y, Kondo Y, Ryousuke U, Yamanaka S (2005) Formation of a new ester compound between triglyceride and dicarboxylic acid catalyzed by lipase. J Mol Catal B Enzym 35:52–56
Acknowledgement
We thank Nestec Ltd. for its financial support to this work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pfeffer, J., Richter, S., Nieveler, J. et al. High yield expression of Lipase A from Candida antarctica in the methylotrophic yeast Pichia pastoris and its purification and characterisation. Appl Microbiol Biotechnol 72, 931–938 (2006). https://doi.org/10.1007/s00253-006-0400-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-006-0400-z