Abstract
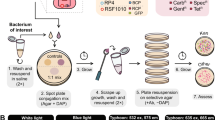
Indigenous gut bacteria of the Formosan subterranean termite (Coptotermes formosanus Shiraki, Isoptera: Rhinotermitidae) were used as shuttle systems to deliver, express and spread foreign genes in termite colonies. The gut bacterium Enterobacter cloacae was transformed with a recombinant plasmid (pEGFP) containing genes encoding ampicillin resistance and green fluorescent protein (GFP). In laboratory experiments, termite workers and soldiers from three colonies were fed with filter paper inoculated with transformed bacteria. Transformed bacteria were detected in termite guts by growing the entire gut flora under selective conditions and checking the cultures visually for fluorescence. We demonstrated that (1) transformed bacteria were ingested within a few hours and the GFP gene was expressed in the termite gut; (2) transformed bacteria established a persistent population in the termite gut for up to 11 weeks; (3) transformed bacteria were efficiently transferred throughout a laboratory colony, even when the donor (termites initially fed with transformed bacteria) to recipient (not fed) ratio was low; (4) transformed E. cloacae were transferred into soil; however, they did not accumulate over time and the GFP plasmid was not transferred to other soil bacteria. In the future, transgenic bacteria may be used to shuttle detrimental genes into termite colonies for improved pest control.






Similar content being viewed by others
References
Armstrong JL, Wood ND, Porteous LA (1990) Transconjugation between bacteria in the digestive tract of the cutworm Peridroma saucia. Appl Environ Microbiol 56:1492–1493
Bextine B, Lauzon C, Potter S, Lampe D, Miller TA (2004) Delivery of a genetically marked Alcaligenes sp. to the glassy-winged sharpshooter for use in a paratransgenic control strategy. Curr Microbiol 48:327–331
Breznak JA (2000) Ecology of prokaryotic microbes in the guts of wood- and litter-feeding termites. In: Abe T, Bignell DE, Higashi M (eds) Termites: evolution, sociality, symbioses, ecology. Kluwer, Dordrecht, pp 209–231
Chao WL, Feng RL (1990) Survival of genetically engineered Escherichia coli in natural soil and river water. J Appl Bacteriol 68:319–325
Chapco W, Kelln RA (1994) Persistence of ingested bacteria in the grasshopper gut. J Invertebr Pathol 64:149–150
Dillon RJ, Dillon VM (2004) The gut bacteria of insects: nonpathogenic interactions. Annu Rev Entomol 49:71–92
Durvasula RV, Sundaram RK, Cordon-Rosales C, Pennington P, Beard BC (2003) Rhodnius prolixus and its symbiont, Rhodococcus rhodnii: a model for paratransgenic control of disease transmission, In: Bourtzis K, Miller TA (eds) Insect symbiosis. CRC, Boca Raton, pp 83–95
Grassé P-P (1949) Ordre de Isoptères ou Termites. In: Grassé P-P (ed) Traité de zoologie, vol IX. Masson, Paris, pp 408–544
Haverty MI (1977) The proportions of soldiers in termite colonies: a list and a bibliography. Sociobiology 2:199–216
Higashiguchi DT, Husseneder C, Berestecky JM, Grace JK (2002) Composition of the culture dependant microbial gut flora of the Formosan subterranean termite. In: Proceedings of the 102nd general meeting of the American Society for Microbiology, Salt Lake City, Utah, May 19–23, 2002, p 76
Hoffmann A, Thimm T, Droge M, Moore ERB, Munch JC, Tebbe CC (1998) Intergeneric transfer of conjugative and mobilizable plasmids harbored by Escherichia coli in the gut of the soil microarthropod Folsomia candida (Collembola). Appl Environ Microbiol 64:2652–2659
Husseneder C, Grace JK, Oishi DE (2005). Use of genetically engineered Escherichia coli to monitor ingestion, loss and transfer of bacteria in termites. Curr Microbiol (in press)
Kuzina LV, Miller ED, Ge B, Miller TA (2002) Transformation of Enterobacter gergoviae isolated from pink bollworm (Lepidoptera: Gelechiidae) gut with Bacillus thuringiensis toxin. Curr Microbiol 44:1–4
La Fage JP, Nutting WL (1978) Nutrient dynamics of termites. In: Brian MV (ed) Production ecology of ants and termites. Cambridge Univ Press, London, pp 165–232
Leff LG, Leff AA (1996) Use of green fluorescent protein to monitor survival of genetically engineered bacteria in aquatic environments. Appl Environ Microbiol 62:3486–3488
Peloquin JJ, Kuzina LV, Lauzon CR, Miller TA (2000) Transformation of internal extracellular bacteria isolated from Rhagoletis completa Cresson gut with enhanced green fluorescent protein. Curr Microbiol 40:367–371
Peloquin JJ, Lauzon CR, Potter SE, Miller TA (2002) Transformed bacterial symbionts reintroduced to and detected in host gut. Curr Microbiol 45:41–45
Su N-Y, Tamashiro M, Yates JR, Haverty MI (1984). Foraging behavior of the Formosan subterranean termite (Isoptera: Rhinotermitidae). Environ Entomol 13:1466–1470
Thimm T, Hoffmann A, Borkott H, Munch JC, Tebbe CC (1998) The gut of the soil microarthropod Folsomia candida (Collembola) is a frequently changeable but selective habitat and a vector for microorganisms. Appl Environ Microbiol 64:2660–2669
Valdivia RH, Hromockyi AE, Monack D, Ramakrishnan L, Falkow S (1996) Applications for green fluorescent protein (GFP) in the study of host–pathogen interactions. Gene 173:47–52
Veivers PC, O’Brien RW, Slaytor M (1982) Role of bacteria in maintaining the redox potential in the hindgut of termites and preventing the entry of foreign bacteria. J Insect Physiol 28:947–951
Watanabe K, Sato M (1998) Plasmid mediated gene transfer between insect resident bacteria, Enterobacter cloacae, and plant-epiphytic bacteria, Erwinia herbicola, in guts of silkworm larvae. Curr Microbiol 37:352–355
Watanabe K, Abe K, Sato M (2000) Biological control of an insect pest by gut-colonizing Enterobacter cloacae transformed with ice nucleation gene. J Appl Microbiol 88:90–97
Acknowledgements
This study was partially supported by USDA-ARS Specific Cooperative Agreements 58-6435-8-107 and 58-6615-9-018. We thank S. Garner and R. Shrestha for technical assistance with the feeding experiments, D. Higashiguchi for bacteria isolation and identification, and Drs. Lane Foil, Jim Ottea and Gregg Henderson for valuable comments on an earlier draft of the manuscript. This is Journal Series No. XXXX of the College of Tropical Agriculture and Human Resources, University of Hawaii. Approved for publication by the Director, Louisiana Agricultural Experiment Station, as Manuscript No. 04-26-0177.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Husseneder, C., Grace, J.K. Genetically engineered termite gut bacteria (Enterobacter cloacae) deliver and spread foreign genes in termite colonies. Appl Microbiol Biotechnol 68, 360–367 (2005). https://doi.org/10.1007/s00253-005-1914-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-005-1914-5