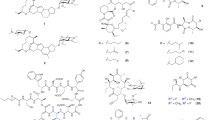
Abstract
Natural products represent an important source of drugs in a number of therapeutic fields, e.g. antiinfectives and cancer therapy. Natural products are considered as biologically validated lead structures, and evolution of compounds with novel or enhanced biological properties is expected from the generation of structural diversity in natural product libraries. However, natural products are often structurally complex, thus precluding reasonable synthetic access for further structure-activity relationship studies. As a consequence, natural product research involves semisynthetic or biotechnological approaches. Among the latter are mutasynthesis (also known as mutational biosynthesis) and precursor-directed biosynthesis, which are based on the cellular uptake and incorporation into complex antibiotics of relatively simple biosynthetic building blocks. This appealing idea, which has been applied almost exclusively to bacteria and fungi as producing organisms, elegantly circumvents labourious total chemical synthesis approaches and exploits the biosynthetic machinery of the microorganism. The recent revitalization of mutasynthesis is based on advancements in both chemical syntheses and molecular biology, which have provided a broader available substrate range combined with the generation of directed biosynthesis mutants. As an important tool in supporting combinatorial biosynthesis, mutasynthesis will further impact the future development of novel secondary metabolite structures.











Similar content being viewed by others
References
Ankenbauer RG, Staley AL, Rinehart KL, Cox CD (1991) Mutasynthesis of siderophore analogues by Pseudomonas aeruginosa. Proc Natl Acad Sci USA 88:1878–1882
Birch AJ (1963) The biosynthesis of antibiotics. Pure Appl Chem 7:527–537
Bormann C, Kalmanczhelyi A, Süssmuth R, Jung G (1999) Production of nikkomycins B x and B z by mutasynthesis with genetically engineered Streptomyces tendae Tü901. J Antibiot 52:102–108
Cane DE, Kudo F, Kinoshita K, Khosla C (2002) Precursor-directed biosynthesis: biochemical basis of the remarkable selectivity of the erythromycin polyketide synthase towards unsaturated triketides. Chem Biol 9:131–142
Cropp TA, Wilson DJ, Reynolds KA (2000) Identification of a cyclohexylcarbonyl CoA biosynthetic gene cluster and application in the production of doramectin. Nat Biotechnol 18:980–983
Daum SJ, Lemke JR (1979) Mutational biosynthesis of new antibiotics. Annu Rev Microbiol 33:241–265
Delzer J, Fiedler, HP, Müller H, Zähner H, Rathmann R, Ernst K, König WA (1984) New nikkomycins by mutasynthesis and directed fermentation. J Antibiot 37:80–82
Dutton CJ, Gibson SP, Goudie AC, Holdom KS, Pacey MS, Ruddock JC (1991) Novel avermectins produced by mutational biosynthesis. J Antibiot 44:357–365
Frykman S, Leaf T, Carreras C, Licari P (2001) Precursor-directedproduction of erythromycin analogs by Saccharopolyspora erythraea.Biotechnol Bioeng 76:303–310
Galm U, Dessoy MA, Schmidt J, Wessjohann LA, Heide L (2004a) In vitro and in vivo production of new aminocoumarins by a combined biochemical, genetic and synthetic approach. Chem Biol 11:173–183
Galm U, Heller S, Shapiro S, Page M, Li S, Heide L (2004b) Antimicrobial and DNA gyrase-inhibitory activities of novel clorobiocin derivatives produced by mutasynthesis. Antimicrob Agents Chemother 48:1307–1312
Graziani EI, Ritacco FV, Summers MY, Zabriskie TM, Yu K, Bernan VS, Greenstein M, Carter GT (2003) Novel sulfur-containing rapamycin analogs prepared by precursor-directed biosynthesis. Org Lett 5:2385–2388
Hafner EW, Holdom KS, Lee SJE (1988) European Patent 0276103
Hojati Z, Milne C, Harvey B, Gordon L, Borg M, Flett F, Wilkinson B, Sidebottom PJ, Rudd BAM, Hayes MA, Smith CP, Micklefield J (2002) Structure, biosynthetic origin, and engineered biosynthesis of calcium-dependent antibiotics from Streptomyces coelicolor. Chem Biol 9:1175–1187
Jacobsen JR, Hutchinson CR, Cane DE, Khosla C (1997) Precursor-directed biosynthesis of erythromycin analogs by an engineered polyketide synthase. Science 277:367–369
Jacobsen JR, Keatinge-Clay AT, Cane DE, Koshla C (1998) Precursor-directed biosynthesis of 12-ethyl erythromycin. Bioorg Med Chem 6:1171–1177
Kalaitzis JA, Izumikawa M, Xiang L, Hertweck C, Moore BS (2003) Mutasynthesis of enterocin and wailupemycin analogues. J Am Chem Soc 125:9290–9291
Kawashima A, Seto H, Kato M, Uchida K, Otake N (1985) Preparation of fluorinated antibiotics followed by fluorine 19F-NMR spectroscopy. I. Fluorinated vulagmycins. J Antibiot 38:1499–1505
Khaw LE, Böhm GA, Metcalfe S, Staunton J, Leadley PF (1998) Mutational biosynthesis of novel rapamycins by a strain of Streptomyces hygroscopicus NRRL 5491 disrupted in rapL, encoding a putative lysine cyclodesaminase. J Bacteriol 180:809–814
Kinoshita K, Williard PG, Khosla C, Cane DE (2001) Precursor-directed biosynthesis of 16-membered macrolides by the erythromycin polyketide sythase. J Am Chem Soc 123:2495–2502
Kinoshita K, Koshla C, Cane DE (2003) Precursor-directed biosynthesis: stereospecificity for branched-chain diketides of the β-ketoacyl-ACP synthase domain 2 of 6-deoxyerythronolide B synthase. Helv Chim Acta 86:3889–3907
Kitamura S, Kase H, Odakura Y, Iida T, Kunikatsu S, Kiyoshi N (1982) 2-Hydroxysagamycin: a new antibiotic produced by mutational biosynthesis of Micromonospora sagamiensis. J Antibiot 35:94–97
Leaf T, Cadapan L, Carreras C, Regentin R, Ou S, Woo E, Ashley G, Licari P (2000) Precursor-directed biosythesis of 6-deoxyerythronolide B analogs in Streptomyces coelicolor: understanding precursor effects. Biotechnol Prog 16:553–556
Marsden AFA, Wilkinson B, Cortes J, Dunster NJ, Staunton J, Leadley PF (1998) Engineering broader specificity into an antibiotic-producing polyketide synthase. Science 279:199–202
McArthur HAI (1998) A novel avermectin, doramectin—a successful application of mutasynthesis. In: Hutchinson CR, McAlpine J (eds) Developments in industrial microbiology—BMP ‘97. Fairfax, Virginia, pp 43–48
Pfeifer V, Nicholson GJ, Ries J, Recktenwald J, Schefer AB, Shawky RM, Schröder J, Wohlleben W, Pelzer S (2001) A polyketide synthase in glycopeptide biosynthesis: the biosynthesis of the non-proteinogenic amino acid (S)-3,5-dihydroxyphenylglycine. J Biol Chem 276:38370–38377
Pieper R, Luo G, Cane DE, Koshla C (1995a) Remarkably broad substrate specificity of a modular polyketide synthase in a cell-free system. J Am Chem Soc 117:11373–11374
Pieper R, Luo G, Cane DE, Koshla C (1995b) Rational design of aromatic polyketide natural products by recombinant assembly of enzymatic subunits. Nature 378:549–554
Puk O, Huber P, Bischoff D, Recktenwald J, Jung G, Süssmuth RD, van Pee KH, Wohlleben W, Pelzer S (2002) Glycopeptide biosynthesis in Amycolatopsis mediterranei DSM5908. Function of a halogenase and a haloperoxidase/perhydrolase. Chem Biol 9:225–235
Rinehart KL (1977) Mutasynthesis of new antibiotics. Pure Appl Chem 49:1361–1384
Shier WT, Rinehart KL, Gottlieb D (1969) Preparation of four new antibiotics from a mutant of Streptomyces fradiae. Proc Natl Acad Sci USA 63:198–204
Süssmuth RD, Wohlleben W (2004) The biosynthesis of glycopeptide antibiotics—a model for complex, non-ribosomally synthesized peptidic secondary metabolites. Appl Microbiol Biotechnol 63:344–350
Takeda K, Kinumaki A, Furumai T, Yamaguchi T, Ohshima S, Ito Y (1978) Mutational biosynthesis of butirosin analogs. J Antibiot 31:247–249
Thiericke R, Rohr J (1993) Biological variation of microbial metabolites by precursor-directed biosynthesis. Nat Prod Rep 10:265–289
Toscano L, Fioriello G, Spagnoli R, Cappelletti L, Zanuso G (1983) New fluorinated erythromycins obtained by mutasynthesis. J Antibiot 36:1439–1450
Weissman KJ, Bycroft M, Cutter AL, Hanefeld U, Frost EJ, Timoney MC, Harris R, Handa S, Roddis M, Staunton J, Leadley PF (1998) Evaluating precursor-directed biosynthesis towards novel erythromycins through in vitro studies on a bimodular polyketide synthase. Chem Biol 5:743–754
Weist S, Bister B, Puk O, Bischoff D, Pelzer S, Nicholson GJ, Wohlleben W, Jung G, Suessmuth RD (2002) Fluorobalhimycin—a new chapter in glycopeptide antibiotic research. Angew Chem 114:3531–3534; Angew Chem Int Ed 41:3383–3385
Weist S, Kittel C, Bischoff D, Bister B, Pfeifer V, Nicholson GJ, Wohlleben W, Süssmuth RD (2004) Mutasynthesis of glycopeptide antibiotics: variations of vancomycin’s AB-ring amino acid 3,5-dihydroxyphenylglycine. J Am Chem Soc 126:5942–5943
Acknowledgements
This work was supported by a grant of the European Union (COMBIG-TOP, LSHG-CT-2003-503491), the Deutsche Forschungsgemeinschaft (DFG, SU 239/3-3) and by an Emmy-Noether-Fellowship for young investigators of the DFG (SU 239/2-1).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Weist, S., Süssmuth, R.D. Mutational biosynthesis—a tool for the generation of structural diversity in the biosynthesis of antibiotics. Appl Microbiol Biotechnol 68, 141–150 (2005). https://doi.org/10.1007/s00253-005-1891-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-005-1891-8