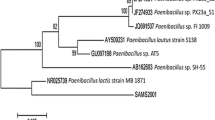
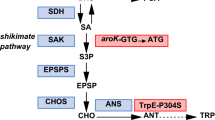
Abstract
The potential of two Rhodococcus strains for biotechnological vanillin production from ferulic acid and eugenol was investigated. Genome sequence data of Rhodococcus sp. I24 suggested a coenzyme A-dependent, non-β-oxidative pathway for ferulic acid bioconversion, which involves feruloyl–CoA synthetase (Fcs), enoyl–CoA hydratase/aldolase (Ech), and vanillin dehydrogenase (Vdh). This pathway was proven for Rhodococcus opacus PD630 by physiological characterization of knockout mutants. However, expression and functional characterization of corresponding structural genes from I24 suggested that degradation of ferulic acid in this strain proceeds via a β-oxidative pathway. The vanillin precursor eugenol facilitated growth of I24 but not of PD630. Coniferyl aldehyde was an intermediate of eugenol degradation by I24. Since the genome sequence of I24 is devoid of eugenol hydroxylase homologous genes (ehyAB), eugenol bioconversion is most probably initiated by a new step in this bacterium. To establish eugenol bioconversion in PD630, the vanillyl alcohol oxidase gene (vaoA) from Penicillium simplicissimum CBS 170.90 was expressed in PD630 together with coniferyl alcohol dehydrogenase (calA) and coniferyl aldehyde dehydrogenase (calB) genes from Pseudomonas sp. HR199. The recombinant strain converted eugenol to ferulic acid. The obtained data suggest that genetically engineered strains of I24 and PD630 are suitable candidates for vanillin production from eugenol.




Similar content being viewed by others
References
Achterholt S, Priefert H, Steinbüchel A (2000) Identification of Amycolatopsis sp. strain HR167 genes, involved in the bioconversion of ferulic acid to vanillin. Appl Microbiol Biotechnol 54:799–807
Altschul SF, Madden TL, Schaffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ (1997) Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res 25:3389–3402
Bell KS, Philp JC, Aw DW, Christofi N (1998) The genus Rhodococcus. J Appl Microbiol 85:195–210
Bentley SD, Chater KF, Cerdeno-Tarraga AM, Challis GL, Thomson NR, James KD, Harris DE, Quail MA, Kieser H, Harper D, Bateman A, Brown S, Chandra G, Chen CW, Collins M, Cronin A, Fraser A, Goble A, Hidalgo J, Hornsby T, Howarth S, Huang CH, Kieser T, Larke L, Murphy L, Oliver K, O’Neil S, Rabbinowitsch E, Rajandream MA, Rutherford K, Rutter S, Seeger K, Saunders D, Sharp S, Squares R, Squares S, Taylor K, Warren T, Wietzorrek A, Woodward J, Barrell BG, Parkhill J, Hopwood DA (2002) Complete genome sequence of the model actinomycete Streptomyces coelicolor A3(2). Nature 417:141–147
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Brandt K, Thewes S, Overhage J, Priefert H, Steinbüchel A (2001) Characterization of the eugenol hydroxylase genes (ehyA/ehyB) from the new eugenol degrading Pseudomonas sp. strain OPS1. Appl Microbiol Biotechnol 56:724–730
Buckland B, Drew S, Connors N, Chartrain M, Lee C, Salmon P, Gbewonyo K, Gailliot P, Singhvi R, Olewinski R, Sun W, Reddy J, Zhang J, Zhou W, Jackey B, Goklen K, Junker B, Greasham R (1999) Microbial conversion of indene to indandiol, a key intermediate in the synthesis of CRIXIVAN. Metab Eng 1:63–74
Bullock WO, Fernandez JM, Stuart JM (1987) XL1-Blue: a high efficiency plasmid transforming recA Escherichia coli strain with beta-galactosidase selection. Biotechniques 5:376–379
Chen CL, Chang HM, Kirk T (1982) Aromatic acids produced during degradation of lignin in spruce wood by Phanerochaete chrysosporium. Holzforschung 36:3–9
da Silva AC, Ferro JA, Reinach FC, Farah CS, Furlan LR, Quaggio RB, Monteiro-Vitorello CB, Van Sluys MA, Almeida NF, Alves LM, do Amaral AM, Bertolini MC, Camargo LE, Camarotte G, Cannavan F, Cardozo J, Chambergo F, Ciapina LP, Cicarelli RM, Coutinho LL, Cursino-Santos JR, El-Dorry H, Faria JB, Ferreira AJ, Ferreira RC, Ferro MI, Formighieri EF, Franco MC, Greggio CC, Gruber A, Katsuyama AM, Kishi LT, Leite RP, Lemos EG, Lemos MV, Locali EC, Machado MA, Madeira AM, Martinez-Rossi NM, Martins EC, Meidanis J, Menck CF, Miyaki CY, Moon DH, Moreira LM, Novo MT, Okura VK, Oliveira MC, Oliveira VR, Pereira HA, Rossi A, Sena JA, Silva C, de Souza RF, Spinola LA, Takita MA, Tamura RE, Teixeira EC, Tezza RI, Trindade dos Santos M, Truffi D, Tsai SM, White FF, Setubal JC, Kitajima JP (2002) Comparison of the genomes of two Xanthomonas pathogens with differing host specificities. Nature 417:459–463
Friedrich B, Hogrefe C, Schlegel HG (1981) Naturally occurring genetic transfer of hydrogen-oxidizing ability between strains of Alcaligenes eutrophus. J Bacteriol 147:198–205
Furukawa H, Wieser M, Morita H, Sugio T, Nagasawa T (1998) Purification and characterization of eugenol dehydrogenase from Pseudomonas fluorescens E118. Arch Microbiol 171:37–43
Gasson MJ, Kitamura Y, McLauchlan WR, Narbad A, Parr AJ, Parsons ELH, Payne J, Rhodes MJC, Walton NJ (1998) Metabolism of ferulic acid to vanillin. A bacterial gene of the enoyl–SCoA hydratase/isomerase superfamily encodes an enzyme for the hydration and cleavage of a hydroxycinnamic acid SCoA thioester. J Biol Chem 273:4163–4170
Goodfellow M, Alderson G, Chun J (1998) Rhodococcal systematics: problems and developments. Antonie Van Leeuwenhoek 74:3–20
Hanahan D (1983) Studies on transformation of Escherichia coli with plasmids. J Mol Biol 166:557–580
Hughes J, Armitage YC, Symes KC (1998) Application of whole cell rhodococcal biocatalysts in acrylic polymer manufacture. Antonie van Leeuwenhoek 74:107–118
Kalscheuer R, Arenskötter M, Steinbüchel A (1999) Establishment of a gene transfer system for Rhodococcus opacus PD630 based on electroporation and its application for recombinant biosynthesis of poly(3-hydroxyalkanoic acids). Appl Microbiol Biotechnol 52:508–515
Knauf VC, Nester EW (1982) Wide host range cloning vectors: a cosmid clone bank of an Agrobacterium Ti plasmid. Plasmid 8:45–54
Kovach ME, Elzer PH, Hill DS, Robertson GT, Farris MA, Roop RM 2nd, Peterson KM (1995) Four new derivatives of the broad host range cloning vector pBBR1MCS, carrying different antibiotic-resistance cassettes. Gene 166:175–176
Krings U, Berger RG (1998) Biotechnological production of flavors and fragrances. Appl Microbiol Biotechnol 49:1–8
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685
Larkin MJ, De Mot R, Kulakov LA, Nagy I (1998) Applied aspects of Rhodococcus genetics. Antonie van Leeuwenhoek 74:133–153
Marmur J (1961) A procedure for the isolation of desoxyribonucleic acids from microorganisms. J Mol Biol 3:208–218
Masai E, Harada K, Peng X, Kitayama H, Katayama Y, Fukuda M (2002) Cloning and characterization of the ferulic acid catabolic genes of Sphingomonas paucimobilis SYK-6. Appl Environ Microbiol 68:4416–4424
Muheim A, Müller B, Münch T, Wetli M (1998) Process for the production of vanillin. European Patent 0885968
Narbad A, Gasson MJ (1998) Metabolism of ferulic acid via vanillin using a novel CoA-dependent pathway in a newly-isolated strain of Pseudomonas fluorescens. Microbiology 144:1397–1405
Nierman WC, Feldblyum TV, Laub MT, Paulsen IT, Nelson KE, Eisen JA, Heidelberg JF, Alley MR, Ohta N, Maddock JR, Potocka I, Nelson WC, Newton A, Stephens C, Phadke ND, Ely B, DeBoy RT, Dodson RJ, Durkin AS, Gwinn ML, Haft DH, Kolonay JF, Smit J, Craven MB, Khouri H, Shetty J, Berry K, Utterback T, Tran K, Wolf A, Vamathevan J, Ermolaeva M, White O, Salzberg SL, Venter JC, Shapiro L, Fraser CM, Eisen J (2001) Complete genome sequence of Caulobacter crescentus. Proc Natl Acad Sci USA 98:4136–4141
O’Brien XM, Parker JA, Lessard PA, Sinskey AJ (2002) Engineering an indene bioconversion process for the production of cis-aminoindanol: a model system for the production of chiral synthons. Appl Microbiol Biotechnol 59:389–399
Overhage J, Priefert H, Rabenhorst J, Steinbüchel A (1999a) Biotransformation of eugenol to vanillin by a mutant of Pseudomonas sp. strain HR199 constructed by disruption of the vanillin dehydrogenase (vdh) gene. Appl Microbiol Biotechnol 52:820–828
Overhage J, Priefert H, Steinbüchel A (1999b) Biochemical and genetic analyses of ferulic acid catabolism in Pseudomonas sp. strain HR199. Appl Environ Microbiol 65:4837–4847
Overhage J, Steinbüchel A, Priefert H (2003) Highly efficient biotransformation of eugenol to ferulic acid and further conversion to vanillin in recombinant strains of Escherichia coli. Appl Environ Microbiol 69:6569–6576
Plaggenborg R, Steinbüchel A, Priefert H (2001) The coenzyme A-dependent, non-β-oxidation pathway and not direct deacetylation is the major route for ferulic acid degradation in Delftia acidovorans. FEMS Microbiol Lett 205:9–16
Plaggenborg R, Overhage J, Steinbüchel A, Priefert H (2003) Functional analyses of genes involved in the metabolism of ferulic acid in Pseudomonas putida KT2440. Appl Microbiol Biotechnol 61:528–535
Priefert H, Rabenhorst J, Steinbüchel A (1997) Molecular characterization of genes of Pseudomonas sp. strain HR199 involved in bioconversion of vanillin to protocatechuate. J Bacteriol 179:2595–2607
Priefert H, Overhage J, Steinbüchel A (1999) Identification and molecular characterization of the eugenol hydroxylase genes (ehyA/ehyB) of Pseudomonas sp. strain HR199. Arch Microbiol 172:354–363
Priefert H, Rabenhorst J, Steinbüchel A (2001) Biotechnological production of vanillin. Appl Microbiol Biotechnol 56:296–314
Rabenhorst J, Hopp R (1997) Process for the preparation of vanillin and suitable microorganisms. European Patent 0761817
Rabenhorst J, Steinbüchel A, Priefert H, Achterholt S (2003) Method for transforming Amycolatopsis sp. DSM9991 and DSM9992. Patent application WO03023017
Salanoubat M, Genin S, Artiguenave F, Gouzy J, Mangenot S, Arlat M, Billault A, Brottier P, Camus JC, Cattolico L, Chandler M, Choisne N, Claudel-Renard C, Cunnac S, Demange N, Gaspin C, Lavie M, Moisan A, Robert C, Saurin W, Schiex T, Siguier P, Thebault P, Whalen M, Wincker P, Levy M, Weissenbach J, Boucher CA (2002) Genome sequence of the plant pathogen Ralstonia solanacearum. Nature 415:497–502
Sambrook J, Fritsch EF, Maniatis T (1989) Molecular cloning: a laboratory manual, 2nd edn. Cold Spring Harbor Laboratory, Cold Spring Harbor
Sanger F, Nicklen S, Coulson AR (1977) DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci USA 74:5463–5467
Schlegel HG, Kaltwasser H, Gottschalk G (1961) Ein Submersverfahren zur Kultur wasserstoffoxidierender Bakterien: Wachstumsphysiologische Untersuchungen. Arch Mikrobiol 38:209–222
Simon R, Priefer U, Pühler A (1983) A broad host range mobilization system for in vivo genetic engineering: transposon mutagenesis in Gram-negative bacteria. Biotechnology (NY) 1:784–791
Tadasa K, Kayahara H (1983) Initial steps of eugenol degradation pathway of a microorganism. Agric Biol Chem 47:2639–2640
Tatusova TA, Madden TL (1999) Blast 2 sequences—a new tool for comparing protein and nucleotide sequences. FEMS Microbiol Lett 174:247–250
Toms A, Wood JM (1970) The degradation of trans-ferulic acid by Pseudomonas acidovorans. Biochemistry 9:337–343
Warhurst AM, Fewson CA (1994) Biotransformations catalyzed by the genus Rhodococcus. Crit Rev Biotechnol 14:29–73
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Plaggenborg, R., Overhage, J., Loos, A. et al. Potential of Rhodococcus strains for biotechnological vanillin production from ferulic acid and eugenol. Appl Microbiol Biotechnol 72, 745–755 (2006). https://doi.org/10.1007/s00253-005-0302-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-005-0302-5