Abstract
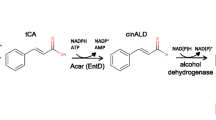
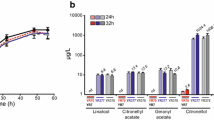
Ditaxis heterantha, a plant of the Euphorbiaceae family, is growing wild in the semiarid regions of Mexico. The seed endosperm contains yellow pigments (carotenoids). By high-pressure liquid chromatography the total pigment (TP) was separated into seven fractions: two of them, heterathin (F4) and ditaxin (F5), characterized as apocarotenoids, represent 80% of TP. Both molecules have double bonds, which seem to be the target for degradation and aroma formation. In this work, TP, F4, and F5 were supplied to nine cultures able to degrade lutein. From these strains, only one (identified as Saccharomyces cerevisiae) was able to produce aromas from either TP or F4. Using TP as substrate, the produced aromas were 4-oxo-isophorone (1), isophorone (2), cinnamic aldehyde (6), 3-hydroxy-β-cyclocitral (7), safranal (8), geranyl (9), 3-oxo-α-ionone (10), 3-oxo-α-ionol (11), 3-oxo-7,8-dihydro-α-ionone (12), and eugenol (13). Of these aromas, only seven were produced from F4: (1), (2), (7), (8), (10), (11), and (12). In both cases, safranal was the main degradation product (30%). The enzymatic activity responsible for this effect was found in the cytosolic fraction and detected only when S. cerevisiae was grown in the presence of TP or F4.




Similar content being viewed by others
References
Belitz HD, Grosch W, Schieberle P (2004) Food chemistry, 3rd edn. Springer, Berlin Heidelberg New York
Bosser A, Belin JM (1994) Synthesis of β-ionone in an aldehyde/xanthine oxidase/β-carotene system involving free radical formation. Biotechnol Prog 10:129–133
Bosser A, Paplorey E, Beling JM (1995) A simple way to (±)-dihydroactinidiolide from β-ionone related to enzymatic co-oxidation of β-carotene in aqueous solution. Biotechnol Prog 11:689–692
Crouzet JC, Kanasawud P (1992) Formation of volatile compounds by thermal degradation of carotenoids. Methods Enzymol 213:54–62
Deli J, Molnár P, Matus Z, Tóth G (2001) Carotenoid composition in the fruits of red paprika (Capsicum annuum var. Lycopersciforme rubrum) during ripening. Biosynthesis of carotenoids in red paprika. J Agric Food Chem 49(3):1517–1523
Enzell C (1985) Biodegradation of carotenoids: an important route to aroma compounds. Pure Appl Chem 57:693–700
Hui YH (1992) Volatile compounds of carotenoids. In: encyclopedia of food and technology, vol 2. Wiley, New York pp 955–964
Jarén-Galán M, Mínguez-Mosquera I (1999) Quantitative and qualitative changes associated with heat treatments in the carotenoid content of paprika oleoresins. J Agric Food Chem 47(10):4379–4383
Knapp H, Straubinger M, Witte A, Winterhalter P (2000) Frontiers of flavour science. In: Schieberle P, Engel KH (eds) Proceedings of the 9th Weurman Flavour Symposium. Eigenverlag Deutsche Forschungsanstalt für Lebensmittelchemie, Garching, Germany, pp 440–444
Kosteridis Y, Razungles A, Bertrand A, Baumes R (2000) Differentiation of the aromas of Merlot and Cabernet Sauvignon wines using sensory and instrumental analysis. J Agric Food Chem 48:5383–5388
Krasnobajew V, Helminger D (1982) Fermentation of fragrances: biotransformation of β-ionone by Lasiodiploida theobromae. Helv Chim Acta 65:1590–1595
Larena I, Salazar O, González V, Julián M, Rubio V (1999) Design of a primer for ribosomal DNA internal transcribed spacer with enhanced specificity for ascomycetes. J Biotechnol 75:187–191
Legler G, Müller-Platz CM, Mentges-Hettkamp M, Pflieger G, Jülich E (1985) On the chemical basis of the Lowry protein determination. Anal Biochem 150:278–287
Lott JA, Turner K (1975) Evaluation of Trinder's glucose oxidase method for measuring glucose in serum and urine. Clin Chem 21:1754–1760
Lutz-Wahl S, Fischer P, Schmidt-Dannert C, Wohlleben W, Hauer B, Schmid RD (1998) Stereo and regioselective hydroxylation of α-ionone by Streptomyces strains. Appl Environ Microbiol 64:3878–3881
Méndez-Robles MD (2004) Identificación y caracterización del pigmento obtenido a partir de las semillas de azafran de bolita (Ditaxis heterantha). Ph.D. thesis, Polytechnic National Institute
Méndez-Robles MD, Flores-Chavira C, Jaramillo-Flores ME, Orozco-Avila I, Lugo Cervantes E (2004) Chemical composition and current distribution of Azafran de Bolita (Ditaxis heterantha Zucc. Euphorbiaceae): a food pigment producing plant. Econ Bot 58:530–535
Méndez MD, Jaramillo ME, Lugo CE, Cardador A, Cerda CM, Lopez de la Mary F, Tamariz J, Permady H (2005) Isolation of two new antioxidant compounds C-26 and C-30 apocarotenoids from the seeds of Ditaxis heterantha. J Nat Prod (in press)
Mikami Y, Fukunaga Y, Arita M, Kisaki T (1981) Microbial transformation of β-ionone and β-methylionone. Appl Environ Microbiol 41:610–617
Pfander H, Schurtenberger H (1982) Biosynthesis of C20-carotenoids in Crocus sativus. Phytochemistry 21:1039–1042
Rodriguez-Bustamante E, Maldonado-Robledo G, Ortiz MA, Díaz-Ávalos C, Sanchez S (2005) Bioconversion of lutein using a microbial mixture—maximizing the production of tobacco aroma compounds by manipulation of culture medium. Appl Microbiol Biotechnol 68:174–182
Sambrook J, Fritsch EF, Maniatis T (1989) Molecular cloning: a laboratory manual, 2nd edn. Cold Spring Harbor Laboratory Press, Cold spring Harbor
Sánchez-Contreras A, Jiménez M, Sanchez S (2000) Bioconversion of lutein to products with aroma. Appl Microbiol Biotechnol 54:528–534
Tai CY, Chen BH (2000) Analysis and stability of carotenoids in the flowers of daylily (Hemerocallis disticha) as affected by various treatments. J Agric Food Chem 48(12):5962–5968
Tarantilis P, Polissiow M (1997) Isolation and identification of the aroma components from saffron (Crocus sativa). J Agric Food Chem 45:459–462
Waché Y, Bosser DA, Mai LH, Belin JM (2002) Co-oxidation of β-carotene in biphasic media. J Mol Catal B Enzym 794:1–5
Wahlberg I, Eklund AM (1998) Carotenoids, vol 3: biosynthesis and metabolism. In: Britton G, Liaaen-Jensen, Pfander H (eds) Birkhäuser Verlag, Basel, Switzerland pp 195–216
Winterhalter P (1996) Carotenoid-derived aroma compounds: biogenetic and biotechnological aspects. In: Takeoka GR, Teranishi R, Williams PJ, Kobayasi A (eds) Biotechnology for improved foods and flavours (ACS Symposium Series 637). American Chemical Society, Washington DC, pp 120–123
Winterhalter P, Rouseff RL (2002) Aroma compounds in tobacco. In: Carotenoid-derived aroma compounds. In: Wahlberg I (ed) American Chemical Society, Stockholm, Sweden, pp 131–143
Acknowledgements
This research was financially supported by the Consejo Nacional de Ciencia y tecnología (CONACYT), Simorelos program (grant 1990305012), and Consejo estatal de ciencia y Tecnología de Jalisco (COECYTJAL, grant 1811). We thank Gabriela Maldonado-Robledo, Eduardo Rodríguez-Bustamante, Mirna Estarrón-Espinoza for their technical assistance in the realization of this work and Hector Escalona-Buendia and Peter Knauth for their help with the text.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Del Toro-Sánchez, L., Sánchez, S., Ortiz, M.A. et al. Generation of aroma compounds from Ditaxis heterantha by Saccharomyces cerevisiae . Appl Microbiol Biotechnol 72, 155–162 (2006). https://doi.org/10.1007/s00253-005-0244-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-005-0244-y