Abstract
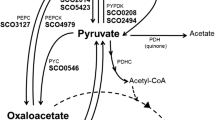
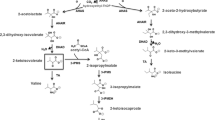
2-Methylcitrate synthase (2-MCS1) and citrate synthase (CS) of Ralstonia eutropha strain H16 were separated by affinity chromatography and analyzed for their substrate specificities. 2-MCS1 used not only the primary substrate propionyl-CoA but also acetyl-CoA and, at a low rate, even butyryl-CoA and valeryl-CoA for condensation with oxaloacetate. The K M values for propionyl-CoA and acetyl-CoA were 0.061 or 0.35 mM, respectively. This enzyme is therefore a competitor for acetyl-CoA during biosynthesis of poly(3-hydroxybutyrate) (PHB) and has to be taken into account if metabolic fluxes are calculated for PHB biosynthesis. In contrast, CS could not use propionyl-CoA as a substrate. The gene-encoding CS (cisY) of R. eutropha was cloned and encodes for a protein consisting of 433 amino acids with a calculated molecular weight of 48,600 Da; it is not truncated in the N-terminal region. Furthermore, a gene encoding a second functionally active 2-methylcitrate synthase (2-MCS2, prpC2) was identified in the genome of R. eutropha. The latter was localized in a gene cluster with genes for an NAD(H)-dependent malate dehydrogenase and a putative citrate lyase. RT-PCR analysis of R. eutropha growing on different carbon sources revealed the transcription of prpC2. In addition, cells of recombinant Escherichia coli strains harboring prpC2 of R. eutropha exhibited high 2-MCS activity of 0.544 U mg−1. A prpC2 knockout mutant of R. eutropha exhibited an identical phenotype as the wild type if grown on different media. 2-MCS2 seems to be dispensable, and a function could not be revealed for this enzyme.





Similar content being viewed by others
References
Altschul SF, Madden TL, Schäffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ (1997) Gapped blast and psi-blast: a new generation of protein database search programs. Nucleic Acids Res 25:3389–3402
Bairoch A, Bucher P, Hoffmann K (1997) The PROSITE database, its status in 1997. Nucleic Acids Res 25:217–221
Birnboim HC, Doly J (1979) A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res 7:1513–1523
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Brämer CO, Steinbüchel A (2001) The methylcitric acid pathway in Ralstonia eutropha: new genes identified involved in propionate metabolism. Microbiology 147:2203–2214
Brämer CO, Steinbüchel A (2002) The malate dehydrogenase of Ralstonia eutropha and functionality of the C3/C4-metabolism in a Tn5-induced mdh mutant. FEMS Microbiol Lett 212:159–164
Brämer CO, Silva LF, Gomez JGC, Priefert H, Steinbüchel A (2002) Identification of the 2-methylcitrate pathway involved in the catabolism of propionate in the polyhydroxyalkanoate producing strain Burkholderia sacchari IPT101T and analysis of a mutant accumulating a copolyester with higher 3-hydroxyvalerate content. Appl Environ Microbiol 68:179–271
Brock M, Buckel W (2004) On the mechanism of action of the antifungal agent propionate: Propionyl-CoA inhibits glucose metabolism in Aspergillus nidulans. Eur J Biochem 271 (15):3227–3241
Brock M, Maerker C, Schutz A, Volker U, Buckel W (2002) Oxidation of propionate to pyruvate in Escherichia coli—involvement of methylcitrate dehydratase and aconitase. Eur J Biochem 269(24):6184–6194
Bullock WO, Fernandez JM, Stuart JM (1987) XL1-Blue: a high efficiency plasmid transforming recAEscherichia coli strain with β-galactosidase selection. BioTechniques 5:376–379
Capela D, Barloy-Hubler F, Gouzy J, Bothe G, Ampe F, Batut J, Boistard P, Becker A, Boutry M, Cadieu E, Dreano S, Gloux S, Godrie T, Goffeau A, Kahn D, Kiss E, Lelaure V, Masuy D, Pohl T, Portetelle D, Pühler A, Purnelle B, Ramsperger U, Renard C, Thebault P, Vandenbol M, Weidner S, Galibert F (2001) Analysis of the chromosome sequence of the legume symbiont Sinorhizobium meliloti strain 1021. Proc Natl Acad Sci U S A 98:9877–9882
Claes WA, Pühler A, Kalinowski J (2002) Identification of two prpDBC gene clusters in Corynebacterium glutamicum and their involvement in propionate degradation via the 2-methylcitrate cycle. J Bacteriol 184:2728–2739
DelVecchio VG, Kapatral V, Redkar RJ, Patra G, Mujer C, Los T, Ivanova N, Anderson I, Bhattacharyya A, Lykidis A, Reznik G, Jablonski L, Larsen N, D'Souza M, Bernal A, Mazur M, Goltsman E, Selkov E, Elzer PH, Hagius S, O'Callaghan D, Letesson J-J, Haselkorn R, Kyrpides N, Overbeek R (2002) The genome sequence of the facultative intracellular pathogen Brucella melitensis. Proc Natl Acad Sci U S A 99:443–448
Donald LJ, Duckworth HW (1987) Expression and base sequence of citrate synthase gene of Acinetobacter anitratum. Biochem Cell Biol 65:930–938
Donald LJ, Molgat CF, Duckworth HW (1989) Cloning, sequencing, and expression of the gene for NADH-sensitive citrate synthase of Pseudomonas aeruginosa. J Bacteriol 171:5542–5550
Ellman GL (1958) A colorimetric method for determining low concentrations of mercaptans. Arch Biochem Biophys 74:443450
Friedrich B, Hogrefe C, Schlegel HG (1981) Naturally occurring genetic transfer of hydrogen-oxidizing ability between strains of Alcaligenes eutrophus. J Bacteriol 147:198–205
Grunstein M, Hogness DS (1975) Colony hybridization: a method for the isolation of cloned DNA that contains a specific gene. Proc Natl Acad Sci U S A 72:3961–3965
Hanahan D (1983) Studies on transformation of Escherichia coli with plasmids. J Mol Biol 166:557–580
Hein S, Steinbüchel A (1994) Biochemical and molecular characterization of the Alcaligenes eutrophus pyruvate dehydrogenase complex and identification of a new type of dihydrolipoamide dehydrogenase. J Bacteriol 176(14):4394–4408
Henderson RA, Jones CW (1997) Poly-3-hydroxybutyrate production by washed cells of Alcaligenes eutrophus; purification, characterization and potential regulatory role of citrate synthase. Arch Microbiol 168:486–492
Hohn B, Collins J (1980) A small cosmid for efficient cloning of large DNA fragments. Gene 11:291–298
Hohn B, Murray K (1977) Packaging recombinant DNA molecules into bacteriophage particles in vitro. Proc Natl Acad Sci U S A 74:3259–3263
Horswill AR, Escalante-Semerena JC (1997) Propionate catabolism in Salmonella typhimurium LT2: two divergently transcribed units comprise the prp locus at 8.5 centisomes, prpR encodes a member of the sigma-54 family of activators, and the prpBCDE genes constitute an operon. J Bacteriol 179:928–940
Kovach ME (1995) Four new derivatives of the broad-host-range cloning vector pBBR1MCS, carrying different antibiotic resistance cassettes. Gene 166:175–176
Kusian B, Bowien B (1997) Organization and regulation of cbb CO2 assimilation genes in autotrophic bacteria. FEMS Microbiol Rev 21:135–155
Marmur J (1961) A procedure for the isolation of desoxyribonucleic acids from microorganism. J Mol Biol 3:208–218
Mothes G, Rivera IS, Babel W (1997) Competition between β-ketothiolase and citrate synthase during poly(β-hydroxybutyrate) synthesis in Methylobacterium rhodesianum. Arch Microbiol 166:405–410
Oelmüller U, Krüger N, Steinbüchel A, Friedrich CG (1990) Isolation of prokaryotic RNA and detection of specific mRNA with biotinylated probes. J Microbiol Methods 11:73–84
Overhage J, Priefert H, Rabenhorst J, Steinbüchel A (1999) Biotransformation of eugenol to vanillin by a mutant of Pseudomonas sp. strain HR199 constructed by disruption of the vanillin dehydrogenase (vdh) gene. Appl Environ Microbiol 52(6):820–828
Overhage J, Steinbüchel A, Priefert H (2002) Biotransformation of eugenol to ferulic acid by a recombinant strain of Ralstonia eutropha H16. Appl Environ Microbiol 68:4315–4321
Palacios S, Escalante-Semerena JC (2000) prpR, ntrA and ihf functions are required for expression of the prpBCDE operon, encoding enzymes that catabolize propionate in Salmonella enterica serovar Typhimurium LT2. J Bacteriol 182:905–910
Palacios S, Escalante-Semerena JC (2004) 2-Methylcitrate-dependent activation of the propionate catabolic operon (prpBCDE) of Salmonella enterica by PrpR protein. Microbiology 150:3877–3887
Pötter M, Müller H, Reinecke F, Wieczorek R, Fricke F, Bowien B, Friedrich B, Steinbüchel (2004) The complex structure of polyhydroxybutyrate (PHB) granules: four orthologous and paralogous phasins occur in Ralstonia eutropha. Microbiology 150:2301–2311
Salanoubat M, Genin S, Artiguenave F, Gouzy J, Mangenot S, Arlat M, Billault A, Brottier P, Camus JC, Cattolico L, Chandler M, Choisne N, Claudel-Renard C, Cunnac S, Demange N, Gaspin C, Lavie M, Moisan A, Robert C, Saurin W, Schiex T, Siguier P, Thebault P, Whalen M, Wincker P, Levy M, Weissenbach J, Boucher CA (2002) Genome sequence of the plant pathogen Ralstonia solanacearum. Nature 415:497–502
Sambrook J, Fritsch EF, Maniatis T (1989) Molecular cloning: a laboratory manual, 2nd edn. Cold Spring Harbor Laboratory, Cold Spring Harbor, NY
Scalenghe F, Turco E, Edström JE, Pirotta V, Melli M (1981) Microdissection and cloning of DNA from specific region of Drosophila melanogaster polytene chromosomes. Chromosoma 82:205–216
Schlegel HG, Kaltwasser H, Gottschalk G (1961) Ein Submersverfahren zur Kultur Wasserstoffoxidierender Bakterien: Wachstumsphysiologische Untersuchungen. Arch Mikrobiol 38:209–222
Schwartz E, Friedrich B (2001) A physical map of the megaplasmid pHG1, one of three replicons in Ralstonia eutropha H16. FEMS Microbiol Lett 201:213–219
Schweizer H, Hoang TT (1995) An improved system for gene replacement and xylE fusion analysis in Pseudomonas aeruginosa. Gene 158:15–22
Simon EJ, Shemin D (1953) The preparation of S-succinyl-coenzyme A. J Am Chem Soc 75:2520–2520
Simon R, Priefer U, Pühler A (1983) A broad host range mobilization system for in vivo genetic engineering: transposon mutagenesis in Gram-negative bacteria. Bio/Technology 1:784–791
Srere PA (1966) Citrate-condensing enzyme-oxaloacetate binary complex. J Biol Chem 241:2157–2165
Srivastava S, Urban M, Friedrich B (1982) Mutagenesis of Alcaligenes eutrophus by insertion of the drug-resistance transposon Tn5. Arch Microbiol 131:203–207
Steinbüchel A (2001) Perspectives for biotechnological production and utilization of biopolymers: metabolic engineering of polyhydroxyalkanoate biosynthesis pathways as a successful example. Macromol Biosci 1:1–24
Steinbüchel A, Pieper A (1992) Production of a copolyester of 3-hydroxybutyric acid and 3-hydroxyvaleric acid from single unrelated carbon sources by a mutant of Alcaligenes eutrophus. Appl Microbiol Biotechnol 37:1–6
Stover CK, Pham XQ, Erwin AL, Mizoguchi SD, Warrener P, Hickey MJ, Brinkman FL, Hufnagle WO, Kowalik DJ, Lagrou M, Garber RL, Goltry L, Tolentino E, Westbrook-Wadman S, Yuan Y, Brody LL, Coulter SN, Folger KR, Kas A, Larbig K, Lim RM, Smith KA, Spencer DH, Wong GKS, Wu Z, Paulsen IT, Reizer J, Saier MH, Hancock REW, Lory S, Olson MV (2000) Complete genome sequence of Pseudomonas aeruginosa PA01, an opportunistic pathogen. Nature 406:959–964
Strauss EC, Kobori JA, Siu G, Hood LE (1986) Specific-primer-directed DNA sequencing. Anal Biochem 154:353–360
Textor S, Wendisch VF, De Graaf AA, Müller U, Linder MI, Linder D, Buckel W (1997) Propionate oxidation in Escherichia coli: evidence for operation of a methylcitrate cycle in bacteria. Arch Microbiol 168:428–436
Uchiyama H, Tabuchi T (1976) Properties of methylcitrate synthase from Candida lipolytica. Agric Biol Chem 40:1411–1418
Yu J, Si Y (2004) Metabolic carbon fluxes and biosynthesis of polyhydroxyalkanoates in Ralstonia eutropha on short chain fatty acids. Biotechnol Prog 20(4):1015–1024
Acknowledgements
We thank Katja Kemper for excellent technical assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ewering, C., Brämer, C.O., Bruland, N. et al. Occurrence and expression of tricarboxylate synthases in Ralstonia eutropha . Appl Microbiol Biotechnol 71, 80–89 (2006). https://doi.org/10.1007/s00253-005-0099-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-005-0099-2