Abstract
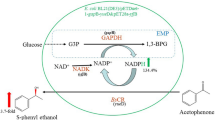
Escherichia coli M15 (pQE30-car0210) was constructed to express carbonyl reductase (CAR) by cloning the car gene from Candida magnoliae and inserting it into pQE30. By cultivating E. coli M15 (pQE30-car0210) and M15 (pQE30-gdh0310), 8.2-fold and 12.3-fold enhancements in specific enzymatic activity over the corresponding original strain were achieved, respectively. After separate cultivations, these two strains were then mixed together at appropriate ratio to construct a novel two-strain system, in which M15 (pQE30-car0210) expressed CAR for ethyl 4-chloro-3-oxobutanoate (COBE) bioreduction and M15 (pQE30-gdh0310) expressed glucose dehydrogenase (GDH) for nicotinamide adenine dinucleotide phosphate (NADPH) regeneration. In this complex system, the effects of substrate concentration, the biomass ratio between two strains as well as reaction temperature were investigated for efficient bioreduction. The results showed that the bioreduction reaction could be completed effectively without any addition of GDH or NADPH/NADP+. An optical purity of 99% (enantiometric efficiency) was obtained, and the yield of (S)-4-chloro-3-hydroxybutanoate ethyl ester reached 96.6% when initial concentration of COBE was 36.9 mM. The coupling reactions between two different strains were further explored by determining the profile of NADPH in the reaction broth.




Similar content being viewed by others
References
Bradford MM (1976) A rapid and sensitive method for the quantization of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Hallinan KO, Crout DHG, Hunt JR, Carter AS, Dalton H, Murrell JC, Holt RA, Crosby J (1995) Yeast catalyzed reduction of β-keto esters(2): optimization of the stereospecific reduction by Zygosaccharomyces rouxii. Biocatal Biotransform 12:179–191
Itamar W, Daniel M (1989) Enzyme-catalyzed biotransformations through photochemical regeneration of nicotinamide cofactors. Enzyme Microb Technol 11:467–483
Jiang B, Liu J-F, Zao S-Y (2001) Enantioselective synthesis for the antipodes of slagenins B and C: establishment of absolute stereochemistry. Org Lett 3:4011–4013
Karanewsky DS, Badia MC, Ciosek CP Jr, Robl JF, Sofia MJ, Simpkins LM, DeLange B, Harrity TW, Biller A, Gordon EM (1990) Phosphorus-containing inhibitors of HMG-CoA reductase I 4-[(2-Arylethyl) hydroxyphosphinyl]-3-hydroxybutanoic acids: a new class of cell selective inhibitors of cholesterol biosynthesis. J Med Chem 33:2952–2956
Kataoka M, Rohani LPS, Yamamoto K, Wada M, Kawabata H, Kita K, Yanase H, Shimizu S (1997) Enzymatic production of ethyl (R)-4-chloro-3-hydroxybutanoate: asymmetric reduction of ethyl 4-chloro-3-oxobutanoate by an Escherichia coli transformant expressing the aldehyde reductase gene from yeast. Appl Microbiol Biotechnol 48:699–703
Kita K, Kataoka M, Shimizu S (1999) Diversity of 4-chloroacetoacetate ethyl ester-reducing enzymes in yeasts and their application to chiral alcohol synthesis. J Biosci Bioeng 88:591–598
Kizaki N, Yasohara Y, Hasegawa J (2001) Synthesis of optically pure ethyl (S)-4-chloro-3-hydrobutanoate by Escherichia coli transformant cells coexpressing the carbonyl reductase and glucose dehydrogenase genes. Appl Microbiol Biotechnol 55:590–595
Liu Y, Xu ZN, Jing KJ, Jiang XX, Lin JP, Cen PL (2004) A novel NADPH-regenerating system for production of chiral alcohols. In: The eighth China–Japan–Korea joint symposium on enzyme engineering, Hangzhou, PR China, 24–28 Oct. 2004, pp 105
Lixin S, Dongzhi W, Siliang Z, Erli W (2001) Studies on synthesis of glutathione by E. coli (pTrc-gsh) coupled with Saccharomyces cerevisiae. Chin J Biotechnol 17:452–455
Patel RN, MeMamee CG, Banerjee A, Howell JM, Robison S, Szarka LJ (1992) Stereoselective reduction of β-keto esters by Geotrichum candidum. Enzyme Microb Technol 14:731–738
Shimizu S, Kataoka M, Katoh M, Morikawa T, Miyoshi T, Yamada H (1990) Stereoselective reduction of ethyl 4-chloro-3-oxobutanoate by a microbial aldehyde reductase in an organic solvent-water diphasic system. Appl Environ Microbiol 56(8):2374–2377
Wade M, Kataoka M, Kawabata H, Yasoharo Y, Kizaki N, Hasegawa J, Shimizu S (1998) Purification and characterization of NADPH-dependent carbonyl reductase, involved in stereoselective reduction of ethyl 4-chloro-3-oxobutanoate from Canadida magnoliae. Biosci Biotechnol Biochem 62:280–285
Walton AZ, Stewart JD (2004) Understanding and improving NADPH-dependent reactions by nongrowing Escherichia coli cells. Biotechnol Prog 20:403–411
Yasoharo Y, Kizaki N, Hasegawa J, Takahashi S, Wada M, Kataoka M, Shimizu S (1999) Synthesis of optically active ethyl 4-chloro-3-hydroxybutanoate by microbial reduction. Appl Microbiol Biotechnol 51:847–851
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Xu, Z., Liu, Y., Fang, L. et al. Construction of a two-strain system for asymmetric reduction of ethyl 4-chloro-3-oxobutanoate to (S)-4-chloro-3-hydroxybutanoate ethyl ester. Appl Microbiol Biotechnol 70, 40–46 (2006). https://doi.org/10.1007/s00253-005-0037-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-005-0037-3