Abstract
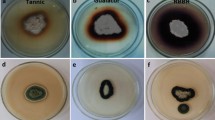
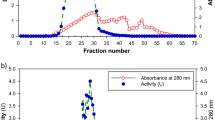
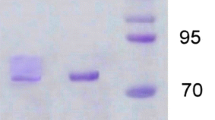
The medicinal mushroom Agaricus blazei produced high amounts of laccase (up to 5,000 units l−1) in a complex, agitated liquid medium based on tomato juice, while only traces of the enzyme (<100 units l−1) were detected in synthetic glucose-based medium. Purification of the enzyme required three chromatographic steps, including anion and cation exchanging. A. blazei laccase was expressed as a single protein with a molecular mass of 66 kDa and an isoelectric point of 4.0. Spectroscopic analysis of the purified enzyme confirmed that it belongs to the “blue copper oxidases”. The enzyme’s pH optimum for 2,6-dimethoxyphenol (DMP) and syringaldazine was pH 5.5; but for 2,2′-azino-bis(3-ethylthiazoline-6-sulfonate) (ABTS) no distinct pH optimum was observed (highest activity at the lowest pH tested). Purified laccase was stable at 20°C, pH 7.0 and pH 3.0, but rapidly lost its activity at 40°C or pH 10. Sodium chloride strongly inhibited the enzyme activity, although the inhibition was completely reversible. The following kinetic constants were determined (Km, kcat): 63 μM, 21 s−1 for ABTS, 4 μM, 5 s−1 for syringaldazine, 1,026 μM, 15 s−1 for DMP and 4307 μM, 159 s−1 for guaiacol. The results show that—in addition to the wood-colonizing white-rot fungi—the typical litter-decomposing basidiomycetes can also produce high titers of laccase in suitable liquid media.






Similar content being viewed by others
References
Arora D (1986) Mushrooms demystified, 2nd edn. Ten Speed, Berkeley
Berger A, Rein D, Kratky E, Monnard I, Hajjaj H, Meirim I, Piguet-Welsch C, Hauser J, Mace K, Niederberger P (2004) Cholesterol-lowering properties of Ganoderma lucidum in vitro, ex vivo, and in hamsters and minipigs. Lipids Health Dis 18:2
Billal F, Thurston CF (1996) Purification of laccase II from Armillaria mellea and comparison of its properties with those of laccase I. Mycol Res 100:1099–1105
Bollag J-M, Sjoblad RD, Liu S-Y (1979) Characterization of an enzyme from Rhizoctonia praticola which polymerizes phenolic compounds. Can J Microbiol 25:229–233
Bonnen AM, Anton LH, Orth AB (1994) Lignin-degrading enzymes of the commercial button mushroom Agaricus bisporus. Appl Environ Microbiol 60:960–965
Borchers AT, Keen CL, Gershwin ME (2004) Mushrooms, tumors, and immunity: an update. Exp Biol Med 229:393–406
Carbajo JM, Junca, Terrón MC, González T, Yagüe S, Zapico E, González AE (2002) Tannic acid induces transcription of laccase gene cglcc1 in the white-rot fungus Coriolopsis gallica. Can J Microbiol 48:1041–1047
Chen S, Ge W, Buswell JA (2004) Biochemical and molecular characterization of a laccase from the edible straw mushroom, Volvariella volvacea. Eur J Biochem 271:318–328
Cohen R, Persky L, Hadar Y (2002) Biotechnological applications and potential of wood-degrading mushrooms of the genus Pleurotus. Appl Microbiol Biotechnol 58:582–594
Eggert C, Temp U, Eriksson KE (1996) The ligninolytic system of the white rot fungus Pycnoporus cinnabarinus: purification and characterization of the laccase. Appl Environ Microbiol 62:1151–1158
Farnet AM, Criquet S, Cigna M, Gil G, Ferre E (2004) Purification of a laccase from Marasmius quercophilus induced with ferulic acid: reactivity towards natural and xenobiotic aromatic compounds. Enzyme Microb Technol 34:549–554
Ghosh A, Frankland JC, Thurston CF, Robinson CH (2003) Enzyme production by Mycena galopus in artificial media and in Picea sichensis F1 horizon needle litter. Mycol Res 107:996–1008
Guterrez ZR, Mantovani MS, Eira AF, Ribeiro LR, Jordao BQ (2004) Variation of the antimutagenicity effects of water extracts of Agaric blazei Murrill in vitro. Toxicol In Vitro 18:301–309
Heinzkill M, Bech L, Halkier T, Schneider P, Anke T (1998) Characterization of laccases and peroxidases from wood-rotting fungi (family Coprinaceae). Appl Environ Microbiol 64:1601–1606
Hofrichter M, Vares T, Kalsi M, Galkin S, Scheibner K, Fritsche W, Hatakka A (1999) Production of manganese peroxidase and organic acids and mineralization of 14C-labelled lignin (14C-DHP) during solid-state fermentation of wheat straw with the white rot fungus Nematoloma frowardii. Appl Environ Microbiol 65:1864–1870
Kim HS, Kacew S, Lee BM (1999) In vitro chemopreventive effects of plant polysaccharides (Aloe barbadensis miller, Lentinus edodes, Ganoderma lucidum and Coriolis versicolor). Carcinogenesis 20:1637–1640
Kimura Y, Kido T, Takaku T, Sumoyoshi M, Baba K (2004) Isolation of an anti-angiogenic substance from Agaricus blazei Murrill: its antitumor and antimetastatic actions. Cancer Sci 95:758–764
Kirk TK, Croan S, Tien M, Murtagh KE, Farrell RL (1986) Production of multiple ligninases by Phanerochaete chrysosporium: effect of selected growth conditions and use of a mutant strain. Enzyme Microb Technol 8:27–32
Kirk PM, Cannon PF, David JC, Stalper JA (eds) (2001) Ainsworth & Bisby’s dictionary of the fungi. CABI Bioscience, Egham
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685
Lankinen VP, Bonnen AM, Anton LH, Wood DA, Kalkkinen N, Hatakka A, Thurston CF (2001) Characteristics and N-terminal amino acid sequence of manganese peroxidase from solid substrate cultures of Agaricus bisporus. Appl Microbiol Biotechnol 55:170–176
Leonowicz A, Grzywnowicz K (1981) Quantitative estimation of laccase forms in some white-rot fungi using syringaldazine as substrate. Enzyme Microb Technol 3:55–58
Mattila P, Salo-Vaananen P, Konko K, Aro H, Jalava T (2002) Basic composition and amino acid contents of mushrooms cultivated in Finland. J Agric Food Chem 50:6419–6422
Munoz C, Guillén F, Martínez AT, Martínez MJ (1997) Induction and characterization of laccase in the ligninolytic fungus Pleurotus eryngii. Curr Microbiol 34:1–5
Murrill WA (1945) New florida fungi. J Fla Acad Sci 8:175–198
Palmieri G, Giardina P, Bianco C, Scaloni A, Capasso A, Sannia G (1997) A novel white laccase from Pleurotus ostreatus. J Biol Chem 272:31301–31307
Pérez J, Rubia T de la, Hamman OB, Martínez J (1998) Phanerochaete flavido-alba laccase induction and modification of manganese peroxidase isoenzyme pattern in decolorized olive oil mill wastewaters. Appl Environ Microbiol 64:2726–2729
Perry CR, Matcham SE, Wood DA, Thurston CF (1993a) The structure of laccase protein and its synthesis by the commercial mushroom A. bisporus. J Gen Microbiol 139:171–178
Perry CR, Smith M, Britnell CH, Wood DA, Thurston CF (1993b) Identification of two laccase genes in the cultivated mushroom A. bisporus. J Gen Microbiol 139:1209–1218
Robene-Soustrade I, Lung-Escarmant B, Bono JJ, Taris B (1992) Identification and partial characterization of an extracellular manganese-dependent peroxidase in Armillaria ostoyae and Armillaria mellea. Eur J For Pathol 22:227–236
Schlosser D, Höfer C (2002) Laccase-catalyzed oxidation of Mn2+ in the presence of natural Mn3+ chelators as a novel source of extracellular H2O2 production and its impact on manganese peroxidase. Appl Environ Microbiol 68:3514–3521
Stamets P. (2000) Growing gourmet and medicinal mushrooms, 3rd edn. Ten Speed, Berkeley
Schneider P, Caspersen MB, Mondorf K, Halkier T, Skov LK, Ostergaard PR, Brown KM, Brown SH, Xu F (1999) Characterization of a Coprinus cinereus Laccase. Enzyme Microb Technol 25:502–508
Steffen KT, Hofrichter M, Hatakka A (2000) Mineralization of 14C-labelled synthetic lignin and ligninolytic enzyme activities of litter-decomposing basidiomycetous fungi. Appl Microbiol Biotechnol 54:819–825
Thurston CF (1994) The structure and function of fungal laccase. Microbiology 140:19–26
Vares T, Kalsi M, Hatakka A (1995) Lignin peroxidases, manganese peroxidases, and other ligninolytic enzymes produced by Phlebia radiata during solid-state fermentation of wheat straw. Appl Environ Microbiol 61:3515–3520
Wariishi H, Valli K, Gold MH (1992) Manganese(II) oxidation by manganese peroxidase from the basidiomycete Phanerochaete chrysosporium. Kinetic mechanism and role of chelator. J Biol Chem 267:23688–23695
Wasser SP (2002) Medicinal mushrooms as a source of antitumor and immunomodulating polysaccharides. Appl Microbiol Biotechnol 60:258–274
Wood DA (1980) Production, purification and properties of extracellular laccase of Agaricus bisporus. J Gen Microbiol 117:327–338
Zhong JJ, Tang YJ (2004) Submerged cultivation of medicinal mushrooms for production of valuable bioactive metabolites. Adv Biochem Eng Biotechnol 87:25–59
Acknowledgements
This work was supported financially by the German Federation of Industrial Cooperative Research Associations “Otto von Guericke” (AiF), the German Academic Exchange Service (DAAD, for L.M.H.), the Academy of Finland (grant 52063, for M.H.) and the administration of the International Graduate School Zittau (R. Konschak, Zittau, Germany). We thank U. Schneider (Zittau) for excellent technical assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ullrich, R., Huong, L.M., Dung, N.L. et al. Laccase from the medicinal mushroom Agaricus blazei: production, purification and characterization. Appl Microbiol Biotechnol 67, 357–363 (2005). https://doi.org/10.1007/s00253-004-1861-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-004-1861-6