Abstract
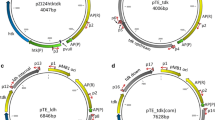
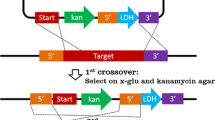
The gene encoding l-lactate dehydrogenase from Thermoanaerobacterium saccharolyticum JW/SL-YS485 was cloned, sequenced, and used to obtain an l-ldh deletion mutant strain (TD1) following a site-specific double-crossover event as confirmed by PCR and Southern blot. Growth rates and final cell densities were similar for strain TD1 and the wild-type grown on glucose and xylose. Lactic acid was below the limit of detection (0.3 mM) for strain TD1 on both glucose and xylose at all times tested, but was readily detected for the wild-type strain, with average final concentrations of 8.1and 1.8 mM on glucose and xylose, respectively. Elimination of lactic acid as a fermentation product was accompanied by a proportional increase in the yields of acetic acid and ethanol. The results reported here represent a step toward using metabolic engineering to develop strains of thermophilic anaerobic bacteria that do not produce organic acids, and support the methodological feasibility of this goal.




Similar content being viewed by others
References
Altaras NE, Cameron DC (1999) Metabolic engineering of a 1,2-propanediol pathway in Escherichia coli. Appl Environ Microbiol 65:1180–1185
Causey TB, Zhou S, Shanmugam KT, Ingram LO (2003) Engineering the metabolism of Escherichia coli W3110 for the conversion of sugar to redox-neutral and oxidized products: Homoacetate production. Proc Natl Acad Sci USA 100:825–832
Desai RP, Papoutsakis ET (1999) Antisense RNA strategies for metabolic engineering of Clostridium acetobutylicum. Appl Environ Microbiol 65:936–945
Green EM, Boynton ZL, Harris LM, Rudolph FB, Papoutsakis ET, Bennett GN (1996) Genetic manipulation of acid formation pathways by gene inactivation in Clostridium acetobutylicum ATCC 824. Microbiology 142:2079–2086
Guedon E, Desvaux M, Petitdemange H (2002) Improvement of cellulolytic properties of Clostridium cellulolyticum by metabolic engineering. Appl Environ Microbiol 68:53–58
Ingram LO, Gomez PF, Lai X, Moniruzzaman M, Wood BE, Yomano LP, York SW (1998) Metabolic engineering of bacteria for ethanol production. Biotechnol Bioeng 58:204–214
Ingram LO, Aldrich HC, Borges ACC, Causey TB, Martinez A, Morales F, Saleh A, Underwood SA, Yomano LP, York SW, Zaldivar J, Zhou SD (1999) Enteric bacterial catalysts for fuel ethanol production. Biotechnol Prog 15:855–866
Liu SY, Gherardini FC, Matuschek M, Bahl H, Wiegel J (1996) Cloning, sequencing, and expression of the gene encoding a large S-layer-associated endoxylanase from Thermoanaerobacterium sp strain JW/SL-YS 485 in Escherichia coli. J Bacteriol 178:1539–1547
Lynd LR, Baskaran S, Casten S (2001) Salt accumulation resulting from base added for pH control, and not ethanol, limits growth of Thermoanaerobacterium thermosaccharolyticum HG-8 at elevated feed xylose concentrations in continuous culture. Biotechnol Prog 17:118–125
Lynd LR, Weimer PJ, van Zyl WH, Pretorius IS (2002) Microbial cellulose utilization: Fundamentals and biotechnology. Microbiol Mol Biol Rev 66:506–577
Mai V, Wiegel J (2000) Advances in development of a genetic system for Thermoanaerobacterium spp: Expression of genes encoding hydrolytic enzymes, development of a second shuttle vector, and integration of genes into the chromosome. Appl Environ Microbiol 66:4817–4821
Mai V, Lorenz WW, Wiegel J (1997) Transformation of Thermoaerobacterium sp Strain JW/SL-YS485 with plasmid pIKM1 conferring kanamycin resistance. FEMS Microbiol Lett 148:163–167
Mat-Jan F, Alam KY, Clark DP (1989) Mutants of Escherichia coli deficient in the fermentative lactate dehydrogenase. J Bacteriol 171:342–348
Nakamura CE, Gatenby AA, Hsu AK-H, La Reau RD, Haynie SL, Diaz-Torres M, Trimbur DE, Whited GM, Nagarajan V, Payne MS, Picataggio SK, Nair RV (January 2000) Method for the production of 1,3-propanediol by recombinant microorganisms. US Patent 6,013,494
Nie W, Draths KM, Frost JW (2002) Benzene-free synthesis of adipic acid. Biotechnol Prog 18:201–211
Papoutsakis ET (1984) Equations and calculations for fermentations of butyric-acid bacteria. Biotechnol Bioeng 26:174–187
Rose TM, Schultz, ER Henikoff JG, Pietrokovski S, McCallum CM, Henikoff S (1998) Consensus-degenerate hybrid oligonucleotide primers for amplification of distantly-related sequences. Nucleic Acids Res 26(7):1628–1635
Sambrook J, Russell DW (eds) (2001) Molecular cloning: a laboratory manual Cold Spring Harbor Laboratory, Cold Spring Harbor, New York
Tao H, Gonzalez R, Martinez A, Rodriguez M, Ingram LO, Preston JF, Shanmugam KT (2001) Engineering a homo-ethanol pathway in Escherichia coli: Increased glycolytic flux and levels of expression of glycolytic genes during xylose fermentation. J Bacteriol 183:2979–2988
Trieu-Cuot P, P Courvalin (1983) Nucleotide sequence of the S faecalis plasmid gene encoding the 3′5′′-aminoglycoside phosphotransferase type III. Gene 23:331–341
Vemuri GN, Eiteman MA, Altman E (2002) Effects of growth mode and pyruvate decarboxylase on succinic acid production by metabolically engineered strains of Escherichia coli. Appl Environ Microbiol 68:1715–1727
Wiegel J, Ljundahl LG, Lawson JR (1979) Isolation from soil and properties of the extreme thermophile Clostridium thermohydrosulfuricum. J Bacteriol 139:800–810
Zhou SD, Causey TB, Hasona A, Shanmugam KT, Ingram LO (2003) Production of optically pure d-lactic acid in mineral salts medium by metabolically engineered Escherichia coli W3110. Appl Environ Microbiol 69:399–407
Acknowledgements
We thank Dr. J. Wiegel for providing us with the wild-type strain Thermoanaerobacterium saccharolyticum JW/SL-YS485 and its genomic DNA library. The support of the National Institute of Standards and Technology (grant no. 60NANB1D0064) and the Link Foundation is gratefully acknowledged. The experiments reported in this study comply with the current laws of the country in which they were performed.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Desai, S.G., Guerinot, M.L. & Lynd, L.R. Cloning of l-lactate dehydrogenase and elimination of lactic acid production via gene knockout in Thermoanaerobacterium saccharolyticum JW/SL-YS485. Appl Microbiol Biotechnol 65, 600–605 (2004). https://doi.org/10.1007/s00253-004-1575-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-004-1575-9