Abstract
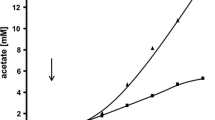
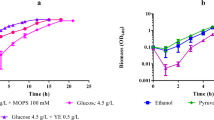
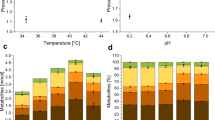
We studied the growth characteristics and oxidative capacities of Acetobacter aceti IFO 3281 in batch and chemostat cultures. In batch culture, glycerol was the best growth substrate and growth on ethanol occurred only after 6 days delay, although ethanol was rapidly oxidized to acetic acid. In continuous culture, both glycerol and ethanol were good growth substrates with similar characteristics. Resting cells in a bioreactor oxidized ribitol to l-ribulose with a maximal specific rate of 1.2 g g−1 h−1). The oxidation of ribitol was inhibited by ethanol but not by glycerol. Biomass yield (YSX; C-mmol/C-mmol) on ethanol and glycerol was low (0.21 and 0.17, respectively). In the presence of ribitol the yield was somewhat higher (0.25) with ethanol but lower (0.13) with glycerol, with respectively lower and higher CO2 production. In chemostat cultures the oxidation rate of ribitol was unaffected by ethanol or glycerol. Cell-free extract oxidized ethanol very slowly but not ribitol; the oxidative activity was located in the cell membrane fraction. Enzymatic activities of some key metabolic enzymes were determined from steady-state chemostat with ethanol, glycerol, or ethanol/glycerol mixture as a growth limiting substrate. Based on the measured enzyme activities, metabolic pathways are proposed for ethanol and glycerol metabolism.




Similar content being viewed by others
References
Adachi O, Fujii Y, Ano Y, Moonmangmee D, Toyama H, Shinagawa E, Theergool G, Lotong N, Matsuhita K (2001) Membrane-bound sugar alcohol dehydrogenase in acetic acid bacteria catalyzes l-ribulose formation and NAD-dependent ribitol dehydrogenase is independent of the oxidative fermentation. Biosci Biotech Biochem 65:115–125
Benziman M, Palgi A (1971) Characterisation and properties of the pyruvate phosphorylation system of Acetobacter xylinum. J Bacteriol 108:211–218
Berg MA van den, Jong-Gubbels P de, Kortland CJ, Dijken JP van, Pronk JT, Steensma HY (1996) The two acetyl-coenzyme A synthetases of Saccharomyces cerevisiae differ with respect to kinetic properties and transcriptional regulation. J Biol Chem 271:28953–28959
Bhuiyan SH, Ahmed Z, Utamura M, Izumori K (1998) A new method for the production of l-lyxose from ribitol using microbial and enzymatic reactions. J Ferment Bioeng 86:513–516
Blomberg A, Adler L (1989) Roles of glycerol and glycerol-3-phosphate dehydrogenase (NAD+) in acquired osmotolerance of Saccharomyces cerevisiae. J Bacteriol 171:1087–1092
Brown DE (1970) Aeration in the submerged culture of microorganisms. (Methods in microbiology vol 2) Academic Press, New York, pp 125–174
Cooper RA, Kornberg HL (1969) Phosphoenolpyruvate synthetase. (Methods in enzymology vol XIII) Academic Press, New York, pp 309–314
Flückiger R, Ettlinger L (1977) Glucose metabolism in Acetobacter aceti. Arch Microbiol 114:183–187
Jokela J, Pastinen O, Leisola M (2001) Isomerization of pentose and hexose sugars by an enzyme reactor packed with cross-linked xylose isomerase crystals. Enzyme Microb Technol 31:67–76
Jong-Gubbels P de, Vanrolleghem P, Heijnen S, Dijken JP van, Pronk JT (1995) Regulation of carbon metabolism in chemostat cultures of Saccharomyces cerevisiae grown on mixtures of glucose and ethanol. Yeast 11:407–418
Jong-Gubbels P de, Dijken JP van, Pronk JT (1996) Metabolic fluxes in chemostat cultures of Schizosaccharomyces pombe grown on mixtures of glucose and ethanol. Microbiol 142:1399–1407
Leisola M, Jokela J, Finell J, Pastinen O (2001) Simultaneous catalysis and product separation by cross-linked enzyme crystals. Biotechnol Bioeng 72:501–505
Lowry OH, Roseborough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265 −275
Maeba P, Sanwall BD (1969) Phosphoenolpyruvate carboxylase from Salmonella typhimurium, strain LT2. (Methods in enzymology vol XIII) Academic Press, New York, pp 283–288
Matsuhita K, Takaki Y, Shinagawa E, Ameyama M, Adachi O (1992) Ethanol oxidase respiratory chain of acetic acid bacteria: reactivity with ubiquinone of pyrroloquinoline quinone-dependent alcohol dehydrogenase purified from Acetobacter aceti and Gluconobacter suboxydans. Biosci Biotechnol Biochem 56:304–310
Nakayama T (1961a) Studies on acetic acid bacteria. III. Purification and properties of coenzyme-independent aldehyde dehydrogenase. J Biochem 49:158–163
Nakayama T (1961b) Studies on acetic acid bacteria. IV. Purification and properties of a new type of alcohol dehydrogenase, alcohol sytochrome-553 reductase. J Biochem 49:240–251
Nakayama T, De Ley J (1965) Localisation and distribution of alcohol-cytochrome 553 reductase in acetic acid bacteria. Antonie van Leeuwenhoek 31:205–219
Neidhardt FC, Ingraham JL, Schaechter M (1990) Physiology of the bacterial cell. Sinauer Associates, Sunderland, Mass.
Nielsen J, Villadsen J (1994) Bioreactor engineering principles. Plenum, New York, p 173
O'Sullivan J, Ettlinger L (1976) Characterization of the acetyl-CoA synthetase of Acetobacter aceti. Biochim Biophys Acta 450:410–417
Outlaw WH Jr, Springer SA (1983) 'Malic' enzyme. (Methods of enzymatic analysis vol III) VCH, Weinheim, pp 176–182
Pastinen O, Visuri K, Schoemaker H, Leisola M (1999) Novel reactions of xylose isomerase from Streptomyces rubiginosus. Enzyme Microb Technol 25:695–700
Postma E, Verduyn C, Scheffers WA, Dijken JP van (1989) Enzymic analysis of the Crabtree effect in glucose-limited chemostat cultures of Saccharomyces cerevisiae. Appl Environ Microbiol 55:468–477
Rao RMR (1955) Pyruvate and acetate metabolism in Acetobacter suboxydans and Acetobacter aceti. Dissertation, University of Illinois, Urbana, Ill.
Reichstein T, Grussner A (1934) Eine ergiebige synthese der l-ascorbinsaure (C-vitamin). Helv Chim Acta 17:996
Scott A (2002) Danisco aims to ramp up sales of 'Pharmaceutical' sugars. Chem Week Nov 20–Nov 27
Smith AF (1983) Malate dehydrogenase. (Methods of enzymatic analysis vol III) VCH, Weinheim, pp 163–176
Stanier RY, Ingraham JL, Wheelis ML, Painter PR (1987) The acetic acid bacteria. In: General microbiology, 5th edn. Macmillan Education, Hong Kong, pp 417–419
Verduyn C (1991) Energetic aspects of metabolic fluxes in yeasts. Dissertation, Delft University of Technology, Delft, The Netherlands
Verduyn C, Kleef R van, Frank J, Schreuder H, Dijken JP van, Scheffers WA (1985) Properties of the NAD(P)H-dependent xylose reductase from the xylose-fermenting yeast Pichia stipitis. Biochem J 226:669–677
Verduyn C, Postma E, Scheffers WA, Dijken JP van (1990) Physiology of Saccharomyces cerevisiae in anaerobic glucose-limited chemostat cultures. J Gen Microbiol 136:395–403
Weber P (1972) Der Einfluss von Glucose auf den Aethanolstoffwechsel eines Stammes von Acetobacter aceti. Dissertation, ETH-Zurich, Switzerland
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kylmä, A.K., Granström, T. & Leisola, M. Growth characteristics and oxidative capacity of Acetobacter aceti IFO 3281: implications for l-ribulose production. Appl Microbiol Biotechnol 63, 584–591 (2004). https://doi.org/10.1007/s00253-003-1406-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-003-1406-4