Abstract
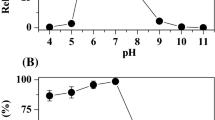
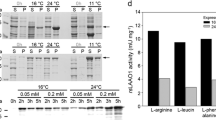
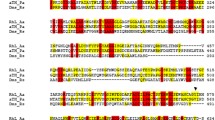
The gene coding for an aerobic azoreductase was cloned from Pigmentiphaga kullae K24, which is able to grow with the carboxylated azo compound 1-(4′-carboxyphenylazo)-4-naphthol (carboxy-Orange I) as sole source of carbon and energy. The gene encoded a protein with a molecular weight of 20,557 Da, with a conserved putative NAD(P)H-binding site in the amino-terminal region. The deduced amino acid sequence showed no further significant sequence homologies to previously studied aerobic azoreductases. The azoreductase was heterologously expressed in Escherichia coli and shown to convert the sulfonated azo dye Orange I and furthermore Magneson II [4-(4-nitrophenylazo)-1-naphthol].

Similar content being viewed by others
References
Alting-Mees MA, Sorge JA, Short JM (1992) pBluescript II: multifunctional cloning and mapping vectors. Methods Enzymol 216:483–495
Anliker R (1979) Ecotoxicology of dyestuffs—a joint effort by industry. Ecotoxicol Environ Saf 3:59–74
Ausubel FM, Brent R, Kingston RE, Moore DD, Seidman JG, Smith JA, Struhl K (1987) Current protocols in molecular biology, vol 1, chapter 2.4. Wiley, New York
Blümel S, Contzen M, Lutz M, Stolz A, Knackmuss H-J (1998) Isolation of a bacterial strain with the ability to utilize the sulfonated azo compound 4-carboxy-4′-sulfoazobenzene as sole source of carbon and energy. Appl Environ Microbiol 64:2315–2317
Blümel S, Busse H-J, Stolz A, Kämpfer P (2001a) Xenophilus azovorans gen. nov., sp. nov., a soil bacterium able to degrade azo dyes of the Orange II type. Int J Syst Evol Bacteriol 51:1831–1837
Blümel S, Mark B, Busse H-J, Kämpfer P, Stolz A (2001b) Pigmentiphaga kullae gen. nov., sp. nov., a new member of the family Alcaligenaceae with the ability to decolorize aerobically azo dyes. Int J Syst Evol Bacteriol 51:1867–1871
Blümel S, Knackmuss H-J, Stolz A (2002) Molecular cloning and characterization of the gene coding for the aerobic azoreductase from Xenophilus azovorans KF46F. Appl Environ Microbiol 68:3948–3955
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Bumpus JA (1995) Microbial degradation of azo dyes. In: Singh VP (ed) Microbial degradation of health risk compounds. Elsevier, Amsterdam, pp 157–167
Coughlin MF, Kinkle BK, Bishop PL (1999) Degradation of azo dyes containing aminonaphthol by Sphingomonas sp. strain 1CX. Ind Microbiol Biotechnol 23:341–346
Inoue H, Nojima H, Okayama H (1990) High efficiency transformation of Escherichia coli with plasmids. Gene 96:23–28
Kulla HG (1981) Aerobic bacterial degradation of azo dyes. In: Leisinger T, Cook AM, Nüesch J, Hütter R (eds) Microbial degradation of xenobiotics and recalcitrant compounds. Academic, London, pp 387–399
Kulla HG, Krieg R, Zimmermann T, Leisinger T (1984) Experimental evolution of azo dye-degrading bacteria. In: Klug MJ, Reddy CA (eds) Current perspectives in microbial ecology. American Society of Microbiology, Washington, D.C., pp 663–667
Marchuk D, Drumm M, Saulino A, Collins FS (1991) Construction of T-vectors, a rapid and general system for direct cloning of unmodified PCR products. Nucleic Acids Res 19:1154
Meehan C, Bjourson AJ, McMullan G (2001) Paenibacillus azoreducens sp. nov., a synthetic azo dye decolorizing bacterium from industrial wastewater. Int J Syst Evol Microbiol 51:1681–1685
Nakanishi M, Yatome C, Ishida N, Kitade Y (2001) Putative ACP phosphodiesterase gene (acpD) encodes an azoreductase. J Biol Chem 276:46394–46399
Sambrook J, Fritsch EF, Maniatis T (1989) Molecular cloning: a laboratory manual, 2nd edn. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
Stolz A (2001) Basic and applied aspects in the microbial degradation of azo dyes. Appl Microbiol Biotechnol 56:69–80
Studier FW, Moffatt BA (1986) Use of bacteriophage T7 RNA polymerase to direct selective high-level expression of cloned genes. J Mol Biol 189:113–130
Suzuki Y, Yoda T, Ruhul A, Sugiura W (2001) Molecular cloning and characterization of the gene coding for azoreductase from Bacillus sp. OY1-2 isolated from soil. J Biol Chem 276:9059–9065
Zimmermann T, Kulla HG, Leisinger T (1982) Properties of purified Orange II azoreductase, the enzyme initiating azo dye degradation by Pseudomonas KF46. Eur J Biochem 129:197–203
Zimmermann T, Gasser F, Kulla HG, Leisinger T (1984) Comparison of two azoreductases acquired during adaptation to growth on azo dyes. Arch Microbiol 138:37–43
Zollinger H (1991) Color chemistry, 2nd edn. VCH, Weinheim
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Blümel, S., Stolz, A. Cloning and characterization of the gene coding for the aerobic azoreductase from Pigmentiphaga kullae K24. Appl Microbiol Biotechnol 62, 186–190 (2003). https://doi.org/10.1007/s00253-003-1316-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-003-1316-5