Abstract
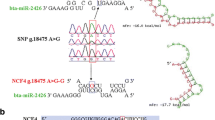
High-mobility group box protein 1 (HMGB1) gene has a universal sentinel function for nucleic acid-mediated innate immune responses and acts as a pathogenic mediator in the inflammatory disease. In an effort to identify the functional single-nucleotide polymorphism (SNP) in the 3′-untranslated region (UTR) of the bovine HMGB1 gene that affects the binding to its target microRNA, first, the expression of HMGB1 mRNA in different genotypes and its candidate bta-miR-223 was investigated. Quantitative real-time polymerase chain reaction results showed that the relative expression of HMGB1 mRNA in cows with the genotype GG is significantly higher than those in cows with the genotype AA (P < 0.05). The expression of bta-miR-223 was significantly upregulated by 1.95-fold (P < 0.05) in the bovine mastitis-infected mammary gland tissues compared with that in the healthy tissues. Subsequently, luciferase assay indicated that the HMGB1 expression was directly targeted by bta-miR-223 in human embryo kidney 293 T (HEK 293T) cells. One novel SNP (g. +2776 A > G) in the HMGB1 3′-UTR, altering the binding of HMGB1 and bta-miR-223, was found to be associated with somatic count scores in cows. Taken together, the g. +2776 A > G-GG was an advantageous genotype which can be used as a candidate functional marker for mastitis resistance breeding program.




Similar content being viewed by others
References
Bannerman DD (2009) Pathogen-dependent induction of cytokines and other soluble inflammatory mediators during intramammary infection of dairy cows. J Anim Sci 87:10–25
Bartel DP (2009) MicroRNAs: Target recognition and regulatory functions. Cell 136:215–233
Chen CZ, Li L, Lodish HF, Bartel DP (2004) MicroRNAs modulate hematopoietic lineage differentiation. Science 303:83–86
Fazi F, Rosa A, Fatica A, Gelmetti V, De Marchis ML et al (2005) A minicircuitry comprised of microRNA-223 and transcription factors NFI-A and C/EBPalpha regulates human granulopoiesis. Cell 123:819–831
Fulci V, Scappucci G, Sebastiani GD, Giannitti C, Franceschini D, Meloni F, Colombo T, Citarella F, Barnaba V, Minisola G, Galeazzi M, Macino G (2010) miR-223 is overexpressed in T-lymphocytes of patients affected by rheumatoid arthritis. Hum Immunol 71:206–211
Georges M, Coppieters W, Charlier C (2007) Polymorphic miRNA-mediated gene regulation: contribution to phenotypic variation and disease. Curr Opin Genet Dev 17:166–176
Harris HE, Andersson U, Pisetsky DS (2012) HMGB1: A multifunctional alarmin driving autoimmune and inflammatory disease. Nat Rev Rheumatol 8:195–202
Hou Q, Huang J, Ju Z, Li Q, Li L, Wang C, Sun T, Wang L, Hou M, Hang S, Zhong J (2012) Identification of splice variants, targeted microRNAs and functional single nucleotide polymorphisms of the BOLA-DQA2 gene in dairy cattle. DNA Cell Biol 31:739–744
Huang J, Wang H, Wang C, Li J, Li Q, Hou M, Zhong J (2010) Single nucleotide polymorphisms, haplotypes and combined genotypes of lactoferrin gene and their associations with mastitis in Chinese Holstein cattle. Mol Biol Rep 37:477–483
Huang JM, Ju ZH, Li QL, Hou QL, Wang CF, Li JB, Li RL, Wang LL, Sun T, Hang SQ, Gao YD, Hou MH, Zhong JF (2011a) Solexa sequencing of novel and differentially expressed microRNAs in testicular and ovarian tissues in Holstein cattle. Int J Biol Sci 7:1016–1026
Huang J, Liu L, Wang HM, Zhang CX, Ju ZH, Wang CF, Zhong JF (2011b) Variants and gene expression of TLR2 gene and susceptibility to mastitis in cattle. Asian J Anim Veterinary Sci 6:51–61
Johnnidis JB, Harris MH, Wheeler RT, Stehling-Sun S, Lam MH, Kirak O, Brummelkamp TR, Fleming MD, Camargo FD (2008) Regulation of progenitor cell proliferation and granulocyte function by microRNA-223. Nature 451:1125–1129
Kloosterman WP, Plasterk RHA (2006) The diverse functions of microRNAs in animal development and disease. Dev Cell 11:441–450
Koralov SB, Muljo SA, Galler GR, Krek A, Chakraborty T, Kanellopoulou C, Jensen K, Cobb BS, Merkenschlager M, Rajewsky N, Rajewsky K (2008) Dicer ablation affects antibody diversity and cell survival in the B lymphocyte lineage. Cell 132:860–874
Kosaka N, Izumi H, Sekine K, Ochiya T (2010) microRNA as a new immune-regulatory agent in breast milk. Silence 1:7
Lim LP, Glasner ME, Yekta S, Burge CB, Bartel DP (2003) Vertebrate microRNA genes. Science 299:1540
Lindberg RL, Hoffmann F, Mehling M, Kuhle J, Kappos L (2010) Altered expression of miR-17-5p in CD4+ lymphocytes of relapsing–remitting multiple sclerosis patients. Eur J Immunol 40:888–898
Nash DL, Rogers GW, Cooper JB, Hargrove GL, Keown JF (2003) Heritability of intramammary infections at first parturition and relationships with sire transmitting abilities for somatic cell score, udder type traits, productive life, and protein yield. J Dairy Sci 86:2684–2695
Nicoloso MS, Sun H, Spizzo R, Kim H, Wickramasinghe P, Shimizu M, Wojcik SE, Ferdin J, Kunej T, Xiao L, Manoukian S, Secreto G, Ravagnani F, Wang X, Radice P, Croce CM, Davuluri RV, Calin GA (2010) Single-nucleotide polymorphisms inside microRNA target sites influence tumor susceptibility. Cancer Res 70:2789–2798
O’Connell RM, Rao DS, Chaudhuri AA, Baltimore D (2010) Physiological and pathological roles for microRNAs in the immune system. Nat Rev Immunol 10:111–122
Pil PM, Lippard SJ (1992) Specific binding of chromosomal protein HMG1 to DNA damaged by the anticancer drug cisplatin. Science 256:234–237
Ramkissoon SH, Mainwaring LA, Ogasawara Y et al (2006) Hematopoietic-specific microRNA expression in human cells. Leuk Res 30:643–647
Scaffidi P, Misteli T, Bianchi ME (2002) Release of chromatin protein HMGB1 by necrotic cells triggers inflammation. Nature 418:191–195
Schiraldi M, Raucci A, Muñoz LM, Livoti E, Celona B, Venereau E, Apuzzo T, De Marchis F, Pedotti M, Bachi A, Thelen M, Varani L, Mellado M, Proudfoot A, Bianchi ME, Uguccioni M (2012) HMGB1 promotes recruitment of inflammatory cells to damaged tissues by forming a complex with CXCL12 and signaling via CXCR4. J Exp Med 209:551–563
Tili E, Michaille JJ, Costinean S, Croce CM (2008) MicroRNAs, the immune system and rheumatic disease. Nat Clin Pract Rheumatol 4:534–541
Voll RE, Urbonaviciute V, Herrmann M, Kalden JR (2008) High mobility group box 1 in the pathogenesis of inflammatory and autoimmune diseases. Isr Med Assoc J 10:26–28
Yanai H, Ban T, Wang Z, Choi MK, Kawamura T, Negishi H, Nakasato M, Lu Y, Hangai S, Koshiba R, Savitsky D, Ronfani L, Akira S, Bianchi ME, Honda K, Tamura T, Kodama T, Taniguchi T (2009) HMGB proteins function as universal sentinels for nucleic-acid-mediated innate immune responses. Nature 462:99–103
Yang EJ, Lee W, Ku SK, Song KS, Bae JS (2012) Anti-inflammatory activities of oleanolic acid on HMGB1 activated HUVECs. Food Chem Toxicol 50:1288–1294
Youngerman SM, Saxton AM, Oliver SP, Pighetti GM (2004) Association of CXCR2 polymorphisms with subclinical and clinical mastitis in dairy cattle. J Dairy Sci 87:2442–2448
Zhuang G, Meng C, Guo X, Cheruku PS, Shi L, Xu H, Li H, Wang G, Evans AR, Safe S, Wu C, Zhou B (2012). A novel regulator of macrophage activation: miR-223 in obesity associated adipose tissue inflammation. Circluation 125:2892–2903
Acknowledgments
This study was supported by grants from the National Natural Science Foundation of China (no. 31000543), Major Project of National Transgene in China (2011ZX08007-001), Support Program of the Ministry of Science and Technology, People’s Republic of China (2011BAD19B02, 2011BAD19B04), China Agriculture Research System (CARS-37), Project of Agricultural Fine Breed from the Department of Science and Technology of Shandong Province (2010LZ10-02).
Conflict of interest
The authors have declared that no conflict of interest exists.
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
Fig. S1
Identification of the pMIR-HMGB1 by restriction PCR and enzyme digestion. M, DL2000 DNA marker; 1, 2, PCR products of plasmid with the different alleles at g. +2776; 3, 4, restriction enzyme digestion products of the different plasmids (DOC 58 kb)
Rights and permissions
About this article
Cite this article
Li, L., Huang, J., Zhang, X. et al. One SNP in the 3′-UTR of HMGB1 gene affects the binding of target bta-miR-223 and is involved in mastitis in dairy cattle. Immunogenetics 64, 817–824 (2012). https://doi.org/10.1007/s00251-012-0641-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00251-012-0641-1