Abstract
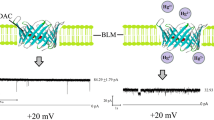
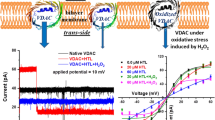
Quinidine is an antiarrhythmic drug commonly used for the treatment of cardiac ailments. It affects oxidative phosphorylation, calcium uptake, and ion channels of mitochondria. We have investigated the interaction of Quinidine and mitochondrial voltage-dependent anion channel (VDAC). VDAC was purified from neuronal tissue of Wistar rats and in vitro bilayer electrophysiology experiments were performed on it. 50-mM Quinidine treatment on VDAC leads to a sudden drop in its conductance. The dose of Quinidine leading to a half-maximal current through a single-channel VDAC was calculated using Quinidine at different concentrations. In silico molecular docking studies using Autodock-4.2 software indicate interaction between Quinidine and VDAC. Docking results demonstrate the interaction of Quinidine and VDAC on its Glutamic acid residue (Glu-206 of VDAC). Fluorescence spectroscopy results on Quinidine and Glutamic acid interaction show an increase in the intensity and wavelength of Quinidine fluorescence, whereas no interaction between Quinidine and Cysteine was observed. This further supports the Glutamic acid and Quinidine interaction. In conclusion, we report Quinidine partially blocks VDAC due to the interaction of Glutamic acid and Quinidine in the channel pore.







Similar content being viewed by others
References
Ackerman MJ (1998) The long QT syndrome: ion channel diseases of the heart. Mayo Clin Proc 73:250–269
Almotrefi AA (1993) Effects of class I antiarrhythmic drugs on mitochondrial ATPase activity in guinea pig heart preparations. Gen Pharmac Vasc Syst 24:233–237
Bachmann E, Weber E, Zbinden G (1986) Biochemical mechanisms of quinidine cardiotoxicity. J Cardiovasc Pharmacol 8:826–831
Banerjee J, Ghosh S (2004a) Bax increases the pore size of rat brain mitochondrial voltage-dependent anion channel in the presence of tBid. Biochem Biophys Res Commun 323:310–314
Banerjee J, Ghosh S (2004b) Interaction of mitochondrial voltage-dependent anion channel from rat brain with plasminogen protein leads to partial closure of the channel. Biochim Biophys Acta 1663:6–8
Bayrhuber M, Meins T, Habeck M, Becker S, Giller K, Villinger S, Vonrhein C, Griesinger C, Zweckstetter M, Zeth K (2008) Structure of the human voltage-dependent anion channel. Proc Natl Acad Sci USA 105:15370–15375
Bednarczyk P, Kicinska A, Kominkova V, Ondrias K, Dolowy K, Szewczyk A (2004) Quinine inhibits mitochondrial ATP-regulated potassium channel from bovine heart. J Memb Biol 199:63–72
Bera AK, Ghosh S, Das S (1995) Mitochondrial VDAC can be phosphorylated by cyclic AMP-dependent protein kinase. Biochem Biophys Res Commun 209:213–217
Bloomer JC, Woods FR, Haddock RE, Lennard MS, Tucker GT (1992) The role of cytochrome P4502D6 in the metabolism of paroxetine by human liver microsomes. Br J Clin Pharmacol 33:521–523
Cesar MdC, Wilson JE (2004) All three isoforms of the voltage-dependent anion channel (VDAC1, VDAC2, and VDAC3) are present in mitochondria from bovine, rabbit, and rat brain. Arch Biochem Biophys 422:191–196
Chan JCY, Soh ACK, Kioh DYQ, Li J, Verma C, Koh SK, Beuerman RW, Zhou L, Chan ECY (2018) Reactive metabolite-induced protein glutathionylation: a potentially novel mechanism underlying acetaminophen hepatotoxicity. MCP 17:2034–2050
Colombini M (1979) A candidate for the permeability pathway of the outer mitochondrial membrane. Nature 279:643–645
Colombini M (1989) Voltage gating in the mitochondrial channel, VDAC. J Memb Biol 111:103–111
Colombini M (2009) The published 3D structure of the VDAC channel: native or not? Trends in biochemical sciences 34:382–389
Colombini M (2012) VDAC structure, selectivity, and dynamics. Biochim Biophys Acta 1818:1457–1465
De Pinto V, Al Jamal JA, Palmieri F (1993) Location of the dicyclohexylcarbodiimide-reactive glutamate residue in the bovine heart mitochondrial porin. J Biol Chem 268:12977–12982
De Pinto V, Messina A, Accardi R, Aiello R, Guarino F, Tomasello MF, Tommasino M, Tasco G, Casadio R, Benz R, De Giorgi F, Ichas F, Baker M, Lawen A (2003) New functions of an old protein: the eukaryotic porin or voltage dependent anion selective channel (VDAC). Ital J Biochem 52:17–24
De Pinto V, Messina A, Lane DJ, Lawen A (2010) Voltage-dependent anion-selective channel (VDAC) in the plasma membrane. FEBS Lett 584:1793–1799
De Pinto V, Reina S, Guarino F, Messina A (2008) Structure of the voltage dependent anion channel: state of the art. J Bioenerg Biomemb 40:139–147
Diwan JJ (1986) Effect of quinine on mitochondrial K+ and Mg++ flux. Biochem Biophys Res Commun 135:830–836
Gross A, McDonnell JM, Korsmeyer SJ (1999) BCL-2 family members and the mitochondria in apoptosis. Genes Dev 13:1899–1911
Gupta R, Ghosh S (2015) Phosphorylation of voltage-dependent anion channel by c-Jun N-terminal Kinase-3 leads to closure of the channel. Biochem Biophys Res Commun 459:100–106
Gupta R, Ghosh S (2017) Phosphorylation of purified mitochondrial voltage-dependent anion channel by c-Jun N-terminal Kinase-3 modifies channel voltage-dependence. Biochim Open 4:78–87
Halestrap AP, Connern CP, Griffiths EJ, Kerr PM (1997) Cyclosporin A binding to mitochondrial cyclophilin inhibits the permeability transition pore and protects hearts from ischaemia/reperfusion injury. Mol Cell Biochem 174:167–172
Harrow JA, Dhalla NS (1976) Effects of quinidine on calcium transport activities of the rabbit heart mitochondria and sarcotubular vesicles. Biochem Pharmacol 25:897–902
Hodge T, Colombini M (1997) Regulation of metabolite flux through voltage-gating of VDAC channels. J Memb Biol 157:271–279
Israelson A, Abu-Hamad S, Zaid H, Nahon E, Shoshan-Barmatz V (2007) Localization of the voltage-dependent anion channel-1 Ca2+-binding sites. Cell Calc 41:235–244
Israelson A, Zaid H, Abu-Hamad S, Nahon E, Shoshan-Barmatz V (2008) Mapping the ruthenium red-binding site of the voltage-dependent anion channel-1. Cell Calc 43:196–204
Jurgens L, Kleineke J, Brdiczka D, Thinnes FP, Hilschmann N (1995) Localization of type-1 porin channel (VDAC) in the sarcoplasmatic reticulum. Biol Chem Hoppe-Seyler 376:685–689
Keinan N, Tyomkin D, Shoshan-Barmatz V (2010) Oligomerization of the mitochondrial protein voltage-dependent anion channel is coupled to the induction of apoptosis. Mol Cell Biol 30:5698–5709
Komai H, Berkoff HA (1979) Effects of quinidine and propranolol on energy transduction in beef heart mitochondria. Biochem Pharmacol 28:1501–1504
Krasnov GS, Dmitriev AA, Lakunina VA, Kirpiy AA, Kudryavtseva AV (2013) Targeting VDAC-bound hexokinase II: a promising approach for concomitant anti-cancer therapy. Expert Opin Ther Targets 17:1221–1233
Levy S, Azoulay S (1994) Stories about the origin of quinquina and quinidine. J Cardiovasc Electrophysiol 5:635–636
Magri A, Karachitos A, Di Rosa MC, Reina S, Conti Nibali S, Messina A, Kmita H, De Pinto V (2019) Recombinant yeast VDAC2: a comparison of electrophysiological features with the native form. FEBS Open Bio 9:1184–1193
Martinou JC, Youle RJ (2011) Mitochondria in apoptosis: Bcl-2 family members and mitochondrial dynamics. Dev Cell 21:92–101
McCommis KS, Baines CP (2012) The role of VDAC in cell death: friend or foe? Biochim Biophys Acta 1818:1444–1450
Mertins B, Psakis G, Grosse W, Back KC, Salisowski A, Reiss P, Koert U, Essen LO (2012) Flexibility of the N-terminal mVDAC1 segment controls the channel's gating behavior. PloS One 7:e47938
Messina A, Reina S, Guarino F, De Pinto V (2012) VDAC isoforms in mammals. Biochim Biophys Acta 1818:1466–1476
Morris GM, Huey R, Lindstrom W, Sanner MF, Belew RK, Goodsell DS, Olson AJ (2009) AutoDock4 and AutoDockTools4: Automated docking with selective receptor flexibility. J Comput Chem 30:2785–2791
Nahon E, Israelson A, Abu-Hamad S, Shoshan-Barmatz V (2005) Fluoxetine (Prozac) interaction with the mitochondrial voltage-dependent anion channel and protection against apoptotic cell death. FEBS Lett 579:5105–5110
Nakashima RA (1989) Hexokinase-binding properties of the mitochondrial VDAC protein: inhibition by DCCD and location of putative DCCD-binding sites. J Bioenerg Biomemb 21:461–470
Pastorino JG, Hoek JB (2008) Regulation of hexokinase binding to VDAC. J Bioenerg Biomemb 40:171–182
Ransom CB, Sontheimer H (2001) BK channels in human glioma cells. J Neurophysiol 85:790–803
Rimmerman N, Ben-Hail D, Porat Z, Juknat A, Kozela E, Daniels MP, Connelly PS, Leishman E, Bradshaw HB, Shoshan-Barmatz V, Vogel Z (2013) Direct modulation of the outer mitochondrial membrane channel, voltage-dependent anion channel 1 (VDAC1) by cannabidiol: a novel mechanism for cannabinoid-induced cell death. Cell Death Dis 4:e949
Rostovtseva TK, Liu T-T, Colombini M, Parsegian VA, Bezrukov SM (2000) Positive cooperativity without domains or subunits in a monomeric membrane channel. Proc Natl Acad Sci 97:7819
Rui H, Lee KI, Pastor RW, Im W (2011) Molecular dynamics studies of ion permeation in VDAC. Biophys J 100:602–610
Sheldon KL, Maldonado EN, Lemasters JJ, Rostovtseva TK, Bezrukov SM (2011) Phosphorylation of voltage-dependent anion channel by serine/threonine kinases governs its interaction with tubulin. PloS One 6:e25539
Shimizu S, Ide T, Yanagida T, Tsujimoto Y (2000) Electrophysiological study of a novel large pore formed by Bax and the voltage-dependent anion channel that is permeable to cytochrome c. J Biol Chem 275:12321–12325
Shoshan-Barmatz V, Ben-Hail D, Admoni L, Krelin Y, Tripathi SS (2015) The mitochondrial voltage-dependent anion channel 1 in tumor cells. Biochim Biophys Acta 1848:2547–2575
Shoshan-Barmatz V, Gincel D (2003) The voltage-dependent anion channel: characterization, modulation, and role in mitochondrial function in cell life and death. Cell Biochem Biophys 39:279–292
Shoshan-Barmatz V, Krelin Y, Shteinfer-Kuzmine A, Arif T (2017) Voltage-dependent anion channel 1 as an emerging drug target for novel anti-cancer therapeutics. Front Oncol 7:154–154
Stock L, Hosoume J, Treptow W (2017) Concentration-dependent binding of small ligands to multiple saturable sites in membrane proteins. Sci Rep 7:5734
Teijido O, Ujwal R, Hillerdal CO, Kullman L, Rostovtseva TK, Abramson J (2012) Affixing N-terminal alpha-helix to the wall of the voltage-dependent anion channel does not prevent its voltage gating. J Biol Chem 287:11437–11445
Thinnes FP (1992) Evidence for extra-mitochondrial localization of the VDAC/porin channel in eucaryotic cells. J Bioenerg Biomemb 24:71–75
Ujwal R, Cascio D, Colletier JP, Faham S, Zhang J, Toro L, Ping P, Abramson J (2008) The crystal structure of mouse VDAC1 at 2.3 A resolution reveals mechanistic insights into metabolite gating. Proc Natl Acad Sci USA 105:17742–17747
Yagoda N, von Rechenberg M, Zaganjor E, Bauer AJ, Yang WS, Fridman DJ, Wolpaw AJ, Smukste I, Peltier JM, Boniface JJ, Smith R, Lessnick SL, Sahasrabudhe S, Stockwell BR (2007) RAS-RAF-MEK-dependent oxidative cell death involving voltage-dependent anion channels. Nature 447:864–868
Yatani A, Wakamori M, Mikala G, Bahinski A (1993) Block of transient outward-type cloned cardiac K+ channel currents by quinidine. Circ Res 73:351–359
Yu WH, Wolfgang W, Forte M (1995) Subcellular localization of human voltage-dependent anion channel isoforms. J Biol Chem 270:13998–14006
Zelcer N, Huisman MT, Reid G, Wielinga P, Breedveld P, Kuil A, Knipscheer P, Schellens JH, Schinkel AH, Borst P (2003) Evidence for two interacting ligand binding sites in human multidrug resistance protein 2 (ATP binding cassette C2). J Biol Chem 278:23538–23544
Zhang XY, Zhang PY (2016) Mitochondria targeting nano agents in cancer therapeutics. Oncol Lett 12:4887–4890
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendix
Appendix
(See Fig. 8).
Other energetically less favorable models of interaction between Quinidine and VDAC. Three-dimensional representation of VDAC1 (PubMed ID: 2JK4) secondary structure and Ligand (PubChem ID: 441074) showing the region of interaction site (green dots) at a Tyr198 on the pore wall, b Glu62 and Thr63 externally, c Glu68 and Thr69 on the pore mouth, and d Glu192 on the pore mouth. The points of contact between the ligand and the VDAC have been represented by spheres/green dots
Rights and permissions
About this article
Cite this article
Malik, C., Ghosh, S. Quinidine partially blocks mitochondrial voltage-dependent anion channel (VDAC). Eur Biophys J 49, 193–205 (2020). https://doi.org/10.1007/s00249-020-01426-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00249-020-01426-z