Abstract
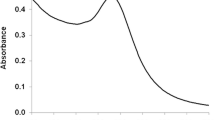
We evaluated the potential of tryptophan (Trp) phosphorescence spectroscopy for investigating conformational states of proteins involved in interaction with nanoparticles. Characterization of protein–nanoparticle interaction is crucial in assessing biological hazards related to use of nanoparticles. We synthesized glutathione-coated CdS quantum dots (GSH-CdS), which exhibited an absorption peak at 366 nm, indicative of 2.4 nm core size. Chemical analysis of purified GSH-CdS suggested an average molecular formula of GSH18S56Cd60. Investigations were conducted on model proteins varying in terms of isoelectric point, degree of burial of the Trp probe, and quaternary structure. GSH-CdS fluorescence measurements showed improvement in nanoparticle quantum yield induced by protein interaction. Trp phosphorescence was used to examine the possible perturbations in the protein native fold induced by GSH-CdS. Phosphorescence lifetime measurements highlighted significant conformational changes in some proteins. Despite their small size, GSH-CdS appeared to interact with more than one protein molecule. Rough determination of the affinity of GSH-CdS for proteins was derived from the change in phosphorescence lifetime at increasing nanoparticle concentrations. The estimated affinities were comparable to those observed for specific protein–ligand interactions and suggest that protein–nanoparticle interaction may have a biological impact.





Similar content being viewed by others
References
Alivisatos AP, Johnsson KP, Peng X, Wilson TE, Loweth CJ, Bruchez MP Jr, Schultz PG (1996) Organization of ‘nanocrystal molecules’ using DNA. Nature 382:609–611
Arai T, Norde W (1990) The behavior of some model proteins at solid liquid interfaces. 2. Sequential and competitive adsorption. Colloids Surf 51:17–28
Bae W, Mehra RK (1998) Properties of glutathione- and phytochelatin-capped CdS bionanocrystallites. J Inorg Biochem 69:33–43
Barglik-Chory C, Buchold D, Schmitt M, Kiefer W, Heske C, Kumpf C, Fuchs O, Weinhardt L, Stahl A, Umbach E, Lentze M, Geurts J, Muller G (2003) Synthesis, structure and spectroscopic characterization of water-soluble CdS nanoparticles. Chem Phys Lett 379:443–451
Bent DV, Hayon E (1975) Excited state chemistry of aromatic amino acids and related peptides. III. Tryptophan. J Am Chem Soc 97:2612–2619
Brown P, Kamat PV (2008) Quantum dot solar cells. Electrophoretic deposition of CdSe-C60 composite films and capture of photogenerated electrons with nC60 cluster shell. J Am Chem Soc 130:8890–8891
Bruchez M Jr, Moronne M, Gin P, Weiss S, Alivisatos AP (1998) Semiconductor nanocrystals as fluorescent biological labels. Science 281:2013–2016
Cedervall T, Lynch I, Lindman S, Berggard T, Thulin E, Nilsson H, Dawson KA, Linse S (2007) Understanding the nanoparticle-protein corona using methods to quantify exchange rates and affinities of proteins for nanoparticles. Proc Natl Acad Sci USA 104:2050–2055
Chen Y, Rosenzweig Z (2002) Luminescent CdS quantum dots as selective ion probes. Anal Chem 74:5132–5138
Chestnoy N, Harris TD, Hull R, Brus LE (1986) Luminescence and photophysics of CdS semiconductor clusters: the nature of the emitting electronic state. J Phys Chem 90:3393–3399
Chothia C, Janin J (1975) Principles of protein–protein recognition. Nature 256:705–708
Cioni P, Strambini GB (1989) Dynamical structure of glutamate dehydrogenase as monitored by tryptophan phosphorescence. Signal transmission following binding of allosteric effectors. J Mol Biol 207:237–247
Cioni P, Strambini GB (2002) Effect of heavy water on protein flexibility. Biophys J 82:3246–3253
Cioni P, Gabellieri E, Gonnelli M, Strambini GB (1994) Heterogeneity of protein conformation in solution from the lifetime of tryptophan phosphorescence. Biophys Chem 52:25–34
De M, You CC, Srivastava S, Rotello VM (2007) Biomimetic interactions of proteins with functionalized nanoparticles: a thermodynamic study. J Am Chem Soc 129:10747–10753
Deliyanni EA, Bakoyannakis DN, Zouboulis AI, Matis KA (2003) Sorption of As(V) ions by akaganeite-type nanocrystals. Chemosphere 50:155–163
Deng ZJ, Liang MT, Monteiro M, Toth I, Minchin RF (2011) Nanoparticle-induced unfolding of fibrinogen promotes Mac-1 receptor activation and inflammation. Nat Nanotechnol 6:39–44
Efros AL, Rodina AV (1989) Confined excitons, trions and biexcitons in semiconductor microcrystals. Solid State Commun 72:435708–435715
Gabellieri E, Strambini GB (1994) Conformational changes in proteins induced by dynamic associations. a tryptophan phosphorescence study. Eur J Biochem 221:77–85
Gabellieri E, Strambini GB (2000) Tryptophan phosphorescence as a monitor of protein conformation in molecular films. Biosens Bioelectron 15:483–490
Gabellieri E, Strambini GB (2001) Structural perturbations of azurin deposited on solid matrices as revealed by trp phosphorescence. Biophys J 80:2431–2438
Gabellieri E, Strambini GB, Shcharbin D, Klajnert B, Bryszewska M (2006) Dendrimer-protein interactions studied by tryptophan room temperature phosphorescence. Biochim Biophys Acta 1764:1750–1756
Gonnelli M, Strambini GB (1995) Phosphorescence lifetime of tryptophan in proteins. Biochemistry 34:13847–13857
Gonnelli M, Strambini GB (2005) Intramolecular quenching of tryptophan phosphorescence in short peptides and proteins. Photochem Photobiol 81:614–622
Handy RD, Owen R, Valsami-Jones E (2008) The ecotoxicology of nanoparticles and nanomaterials: current status, knowledge gaps, challenges, and future needs. Ecotoxicology 17:315–325
Hellstrand E, Lynch I, Andersson A, Drakenberg T, Dahlback B, Dawson KA, Linse S, Cedervall T (2009) Complete high-density lipoproteins in nanoparticle corona. Febs J 276:3372–3381
Hong R, Fischer NO, Verma A, Goodman CM, Emrick T, Rotello VM (2004) Control of protein structure and function through surface recognition by tailored nanoparticle scaffolds. J Am Chem Soc 126:739–743
Hung A, Mwenifumbo S, Mager M, Kuna JJ, Stellacci F, Yarovsky I, Stevens MM (2011) Ordering surfaces on the nanoscale: implications for protein adsorption. J Am Chem Soc 133:1438–1450
Jiang C, Xu SK, Yang DZ, Zhang FH, Wang WX (2007) Synthesis of glutathione-capped US quantum dots and preliminary studies on protein detection and cell fluorescence image. Luminescence 22:430–437
Jiang X, Weise S, Hafner M, Rocker C, Zhang F, Parak WJ, Nienhaus GU (2010) Quantitative analysis of the protein corona on FePt nanoparticles formed by transferrin binding. J R Soc Interface 7(suppl 1):S5–S13
Karlsson BG, Pascher T, Nordling M, Arvidsson RH, Lundberg LG (1989) Expression of the blue copper protein azurin from Pseudomonas aeruginosa in Escherichia coli. FEBS Lett 246:211–217
Lapidus LJ, Eaton WA, Hofrichter J (2001) Dynamics of intramolecular contact formation in polypeptides: distance dependence of quenching rates in a room-temperature glass. Phys Rev Lett 87:258101
Lundqvist M, Sethson I, Jonsson BH (2004) Protein adsorption onto silica nanoparticles: conformational changes depend on the particles’ curvature and the protein stability. Langmuir 20:10639–10647
Lundqvist M, Stigler J, Elia G, Lynch I, Cedervall T, Dawson KA (2008) Nanoparticle size and surface properties determine the protein corona with possible implications for biological impacts. Proc Natl Acad Sci USA 105:14265–14270
Lynch I, Dawson KA (2008) Protein-nanoparticle interactions. Nano Today 3:40–47
Lynch I, Cedervall T, Lundqvist M, Cabaleiro-Lago C, Linse S, Dawson KA (2007) The nanoparticle-protein complex as a biological entity; a complex fluids and surface science challenge for the 21st century. Adv Colloid Interface Sci 134–135:167–174
Lynch I, Salvati A, Dawson KA (2009) Protein-nanoparticle interactions what does the cell see? Nat Nanotechnol 4:546–547
Makarucha AJ, Todorova N, Yarovsky I (2011) Nanomaterials in biological environment: a review of computer modelling studies. Eur Biophys J 40:103–115
Medintz IL, Uyeda HT, Goldman ER, Mattoussi H (2005) Quantum dot bioconjugates for imaging, labelling and sensing. Nat Mater 4:435–446
Michalet X, Pinaud FF, Bentolila LA, Tsay JM, Doose S, Li JJ, Sundaresan G, Wu AM, Gambhir SS, Weiss S (2005) Quantum dots for live cells, in vivo imaging, and diagnostics. Science 307:538–544
Monopoli MP, Bombelli FB, Dawson KA (2011) Nanoparticle coronas take shape. Nat Nanotechnol 6:11–12
Morelli E, Scarano G (2001) Synthesis and stability of phytochelatins induced by cadmium and lead in the marine diatom Phaeodactylum tricornutum. Mar Environ Res 52:383–395
Neder RB, Korsunskiy VI (2005) Structure of nanoparticles from powder diffraction data using the pair distribution function. J Phys Condens Matter 17:S125–S134
Nguyen L, Kho R, Bae W, Mehra RK (1999) Glutathione as a matrix for the synthesis of CdS nanocrystallites. Chemosphere 38:155–173
Rabinowitz JC (1978) Analysis of acid-labile sulfide and sulfhydryl groups. Methods Enzymol 53:275–277
Rocker C, Potzl M, Zhang F, Parak WJ, Nienhaus GU (2009) A quantitative fluorescence study of protein monolayer formation on colloidal nanoparticles. Nat Nanotechnol 4:577–580
Sabatino P, Casella L, Granata A, Iafisco M, Lesci IG, Monzani E, Roveri N (2007) Synthetic chrysotile nanocrystals as a reference standard to investigate surface-induced serum albumin structural modifications. J Colloid Interface Sci 314:389–397
Scarano G, Morelli E (2003) Properties of phytochelatin-coated CdS nanocrystallites formed in a marine phytoplanktonic alga (Phaeodactylum tricornutum, Bohlin) in response to Cd. Plant Sci 165:803–810
Shang W, Nuffer JH, Dordick JS, Siegel RW (2007) Unfolding of ribonuclease A on silica nanoparticle surfaces. Nano Lett 7:1991–1995
Spanhel L, Haase M, Weller H, Henglein A (1987) Photochemistry of colloidal semiconductors. 20. Surface modification and stability of strong luminescing CdS particles. J Am Chem Soc 109:5649–5655
Strambini GB, Gabellieri E (1990) Temperature-dependence of tryptophan phosphorescence in proteins. Photochem Photobiol 51:643–648
Strambini GB, Gabellieri E (1996) Proteins in frozen solutions: evidence of ice-induced partial unfolding. Biophys J 70:971–976
Strambini GB, Gonnelli M (1995) Tryptophan Phosphorescence in fluid solution. J Am Chem Soc 117:7646–7651
Strambini GB, Cioni P, Puntoni A (1989) Relationship between the conformation of glutamate dehydrogenase, the state of association of its subunit, and catalytic function. Biochemistry 28:3808–3814
Strambini GB, Kerwin BA, Mason BD, Gonnelli M (2004) The triplet-state lifetime of indole derivatives in aqueous solution. Photochem Photobiol 80:462–470
Tavares AJ, Chong LR, Petryayeva E, Algar WR, Krull UJ (2011) Quantum dots as contrast agents for in vivo tumor imaging: progress and issues. Anal Bioanal Chem 399:2331–2342
Thangadurai P, Balaji S, Manoharan PT (2008) Surface modification of CdS quantum dots using thiols—structural and photophysical studies. Nanotechnology 19:435708
Wu X, Liu H, Liu J, Haley KN, Treadway JA, Larson JP, Ge N, Peale F, Bruchez MP (2003) Immunofluorescent labeling of cancer marker Her2 and other cellular targets with semiconductor quantum dots. Nat Biotechnol 21:41–46
Yu WW, Qu LH, Guo WZ, Peng XG (2003) Experimental determination of the extinction coefficient of CdTe, CdSe, and CdS nanocrystals. Chem Mater 15:2854–2860
Zou L, Fang Z, Gu ZY, Zhong XH (2009) Aqueous phase synthesis of biostabilizer capped CdS nanocrystals with bright emission. J Lumin 129:536–540
Acknowledgments
The technical assistance of Alessandro Puntoni is duly acknowledged.
Author information
Authors and Affiliations
Corresponding author
Additional information
Special Issue: SIBPA 2011 Meeting.
Rights and permissions
About this article
Cite this article
Gabellieri, E., Cioni, P., Balestreri, E. et al. Protein structural changes induced by glutathione-coated CdS quantum dots as revealed by Trp phosphorescence. Eur Biophys J 40, 1237–1245 (2011). https://doi.org/10.1007/s00249-011-0736-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00249-011-0736-x