Abstract
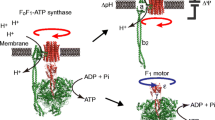
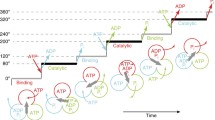
F1-ATPase is a molecular motor in which the γ subunit rotates inside the α3β3 ring upon adenosine triphosphate (ATP) hydrolysis. Recent works on single-molecule manipulation of F1-ATPase have shown that kinetic parameters such as the on-rate of ATP and the off-rate of adenosine diphosphate (ADP) strongly depend on the rotary angle of the γ subunit (Hirono-Hara et al. 2005; Iko et al. 2009). These findings provide important insight into how individual reaction steps release energy to power F1 and also have implications regarding ATP synthesis and how reaction steps are reversed upon reverse rotation. An important issue regarding the angular dependence of kinetic parameters is that the angular position of a magnetic bead rotation probe could be larger than the actual position of the γ subunit due to the torsional elasticity of the system. In the present study, we assessed the stiffness of two different portions of F1 from thermophilic Bacillus PS3: the internal part of the γ subunit embedded in the α3β3 ring, and the complex of the external part of the γ subunit and the α3β3 ring (and streptavidin and magnetic bead), by comparing rotational fluctuations before and after crosslinkage between the rotor and stator. The torsional stiffnesses of the internal and remaining parts were determined to be around 223 and 73 pNnm/radian, respectively. Based on these values, it was estimated that the actual angular position of the internal part of the γ subunit is one-fourth of the magnetic bead position upon stalling using an external magnetic field. The estimated elasticity also partially explains the accommodation of the intrinsic step size mismatch between Fo and F1-ATPase.




Similar content being viewed by others
Notes
The number of molecules after reduction is smaller than before reduction. This is because molecules whose rotation behavior, such as angle distribution or pause position, differed largely from the original ones were omitted from the data analysis.
References
Adachi K, Oiwa K, Nishizaka T, Furuike S, Noji H, Itoh H, Yoshida M, Kinosita K Jr (2007) Coupling of rotation and catalysis in F(1)-ATPase revealed by single-molecule imaging and manipulation. Cell 130:309–321. doi:10.1016/j.cell.2007.05.020
Ariga T, Muneyuki E, Yoshida M (2007) F1-ATPase rotates by an asymmetric, sequential mechanism using all three catalytic subunits. Nat Struct Mol Biol 14:841–846. doi:10.1038/nsmb1296
Bandyopadhyay S, Allison WS (2004) The ionic track in the F1-ATPase from the thermophilic Bacillus PS3. Biochemistry 43:2533–2540. doi:10.1021/bi036058i
Duser MG, Zarrabi N, Cipriano DJ, Ernst S, Glick GD, Dunn SD, Borsch M (2009) 36 degrees step size of proton-driven c-ring rotation in FoF1-ATP synthase. EMBO J 28:2689–2696. doi:10.1038/emboj.2009.213
Enoki S, Watanabe R, Iino R, Noji H (2009) Single-molecule study on the temperature-sensitive reaction of F1-ATPase with a hybrid F1 carrying a single beta(E190D). J Biol Chem 284:23169–23176. doi:10.1074/jbc.M109.026401
Furuike S, Hossain MD, Maki Y, Adachi K, Suzuki T, Kohori A, Itoh H, Yoshida M, Kinosita K Jr (2008) Axle-less F1-ATPase rotates in the correct direction. Science 319:955–958. doi:10.1126/science.1151343
Hirono-Hara Y, Noji H, Nishiura M, Muneyuki E, Hara KY, Yasuda R, Kinosita K Jr, Yoshida M (2001) Pause and rotation of F(1)-ATPase during catalysis. Proc Natl Acad Sci USA 98:13649–13654. doi:10.1073/pnas.241365698
Hirono-Hara Y, Ishizuka K, Kinosita K Jr, Yoshida M, Noji H (2005) Activation of pausing F1 motor by external force. Proc Natl Acad Sci USA 102:4288–4293. doi:10.1073/pnas.0406486102
Iko Y, Tabata KV, Sakakihara S, Nakashima T, Noji H (2009) Acceleration of the ATP-binding rate of F1-ATPase by forcible forward rotation. FEBS Lett 583:3187–3191. doi:10.1016/j.febslet.2009.08.042
Junge W, Sielaff H, Engelbrecht S (2009) Torque generation and elastic power transmission in the rotary F(O)F(1)-ATPase. Nature 459:364–370. doi:10.1038/nature08145
Ma J, Flynn TC, Cui Q, Leslie AG, Walker JE, Karplus M (2002) A dynamic analysis of the rotation mechanism for conformational change in F(1)-ATPase. Structure 10:921–931. doi:10.1016/S0969-2126(02)00789-X
Mitome N, Suzuki T, Hayashi S, Yoshida M (2004) Thermophilic ATP synthase has a decamer c-ring: indication of noninteger 10:3 H+/ATP ratio and permissive elastic coupling. Proc Natl Acad Sci USA 101:12159–12164. doi:10.1073/pnas.0403545101
Noji H, Yasuda R, Yoshida M, Kinosita K Jr (1997) Direct observation of the rotation of F1-ATPase. Nature 386:299–302. doi:10.1038/386299a0
Okuno D, Fujisawa R, Iino R, Hirono-Hara Y, Imamura H, Noji H (2008) Correlation between the conformational states of F1-ATPase as determined from its crystal structure and single-molecule rotation. Proc Natl Acad Sci USA 105:20722–20727. doi:10.1073/pnas.0805828106
Oster G, Wang H (2000) Reverse engineering a protein: the mechanochemistry of ATP synthase. Biochim Biophys Acta 1458:482–510. doi:10.1016/S0005-2728(00)00096-7
Panke O, Rumberg B (1999) Kinetic modeling of rotary CF0F1-ATP synthase: storage of elastic energy during energy transduction. Biochim Biophys Acta 1412:118–128. doi:10.1016/S0005-2728(99)00059-6
Rondelez Y, Tresset G, Nakashima T, Kato-Yamada Y, Fujita H, Takeuchi S, Noji H (2005) Highly coupled ATP synthesis by F1-ATPase single molecules. Nature 433:773–777. doi:10.1038/nature03277
Shimabukuro K, Yasuda R, Muneyuki E, Hara KY, Kinosita K Jr, Yoshida M (2003) Catalysis and rotation of F1 motor: cleavage of ATP at the catalytic site occurs in 1 ms before 40 degree substep rotation. Proc Natl Acad Sci USA 100:14731–14736. doi:10.1073/pnas.2434983100
Sielaff H, Rennekamp H, Wachter A, Xie H, Hilbers F, Feldbauer K, Dunn SD, Engelbrecht S, Junge W (2008) Domain compliance and elastic power transmission in rotary F(O)F(1)-ATPase. Proc Natl Acad Sci USA 105:17760–17765. doi:10.1073/pnas.0807683105
Ueno H, Nishikawa S, Iino R, Tabata KV, Sakakihara S, Yanagida T, Noji H (2010) Simple dark-field microscopy with nanometer spatial precision and microsecond temporal resolution. Biophys J 98:2014–2023. doi:10.1016/j.bpj.2010.01.011
von Ballmoos C, Cook GM, Dimroth P (2008) Unique rotary ATP synthase and its biological diversity. Annu Rev Biophys 37:43–64. doi:10.1146/annurev.biophys.37.032807.130018
Watanabe R, Iino R, Shimabukuro K, Yoshida M, Noji H (2008) Temperature-sensitive reaction intermediate of F1-ATPase. EMBO Rep 9:84–90. doi:10.1038/sj.embor.7401135
Yasuda R, Noji H, Kinosita K Jr, Yoshida M (1998) F1-ATPase is a highly efficient molecular motor that rotates with discrete 120 degree steps. Cell 93:1117–1124. doi:10.1016/S0092-8674(00)81456-7
Yasuda R, Noji H, Yoshida M, Kinosita K Jr, Itoh H (2001) Resolution of distinct rotational sub steps by sub millisecond kinetic analysis of F1-ATPase. Nature 410:898–904. doi:10.1038/35073513
Yoshida M, Muneyuki E, Hisabori T (2001) ATP synthase–a marvellous rotary engine of the cell. Nat Rev Mol Cell Biol 2:669–677. doi:10.1038/35089509
Acknowledgments
We thank Y. Iko-Tabata and R. Hasegawa for technical assistance, W. Allison (University of California, San Diego) for plasmid vectors of mutant TF1 from Bacillus PS3, K. Adachi (Gakushuin University) for the custom image analysis program, and members of the Noji laboratory for help and advice. This work was supported by a Grant-in-Aid for Scientific Research (No. 18074005 to H.N.) from the Ministry of Education, Culture, Sports, Science, and Technology of Japan.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
249_2010_616_MOESM1_ESM.pdf
Typical power spectrum of thermal fluctuation of a magnetic bead at ATP binding position at 3000 frames/s. This spectrum is fitted using a Lorentzian curve (black line). The cutoff frequency is determined to be 29.8 Hz from the fitting parameter, indicated by a broken line
Rights and permissions
About this article
Cite this article
Okuno, D., Iino, R. & Noji, H. Stiffness of γ subunit of F1-ATPase. Eur Biophys J 39, 1589–1596 (2010). https://doi.org/10.1007/s00249-010-0616-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00249-010-0616-9