Abstract
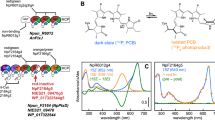
Two phytochromes, CphA and CphB, from the cyanobacterium Calothrix PCC7601, with similar size (768 and 766 amino acids) and domain structure, were investigated for the essential length of their protein moiety required to maintain the spectral integrity. Both proteins fold into PAS-, GAF-, PHY-, and Histidine-kinase (HK) domains. CphA binds a phycocyanobilin (PCB) chromophore at a “canonical” cysteine within the GAF domain, identically as in plant phytochromes. CphB binds biliverdin IXα at cysteine24, positioned in the N-terminal PAS domain. The C-terminally located HK and PHY domains, present in both proteins, were removed subsequently by introducing stop-codons at the corresponding DNA positions. The spectral properties of the resulting proteins were investigated. The full-length proteins absorb at (CphA) 663 and 707 nm (red-, far red-absorbing P r and P fr forms of phytochromes) and at (CphB) 704 and 750 nm. Removal of the HK domains had no effect on the absorbance maxima of the resulting PAS–GAF–PHY constructs (CphA: 663/707 nm, CphB: 704/750 nm, P r/P fr, respectively). Further deletion of the “PHY” domains caused a blue-shift of the P r and P fr absorption of CphA (λ max: 658/698 nm) and increased the amount of unproperly folded apoprotein, seen by a reduced capability to bind the chromophore in photoconvertible manner. In CphB, however, it practically impaired the formation of P fr, i.e., showing a very low oscillator strength absorption band, whereas the P r form remains unchanged (702 nm). This finding clearly indicates a different interaction between domains in the “typical”, PCB binding and in the biliverdin-binding phytochromes, and demonstrates a loss of oscillator strength for the latter, most probably due to a strong conformational distortion of the chromophore in the CphB P fr form.




Similar content being viewed by others
Abbreviations
- BV:
-
Biliverdin IXα
- GAF:
-
Acronym for: cGMP-specific and–regulated cyclic nucleotide phosphodiesterase, Adenylyl cyclase, and E. coli transcription factor FhlA
- PAS (protein domain):
-
PerArntSim (protein domain)
- PCB:
-
Phycocyanobilin
- P r, P fr :
-
Red-, far red absorbing forms of phytochrome
- PHY:
-
Phytochrome-specific protein domain
References
Boylan MT, Quail PH (1991) Phytochrome A overexpression inhibits hypocotyl elongation in transgenic Arabidopsis. Proc Natl Acad Sci USA 88:10806–10810
Boylan MT, Quail PH (1996) Are the phytochromes protein kinases? Protoplasma 195:12–17
Esteban B, Carrascal M, Abian J, Lamparter T (2005) Light-induced changes of cyanobacterial phytochrome Cph1 probed by limited proteolysis and autophosphorylation. Biochemistry 44:450–461
Gärtner W, Braslavsky SE (2003) The phytochromes: spectroscopy and function. In: Batschauer A (ed) Photoreceptors and light signaling. Royal Society of Chemistry, Cambridge, pp 137–180
Gärtner W, Hill C, Worm K, Braslavsky SE, Schaffner K (1996) Influence of expression system on chromophore binding and preservation of spectral properties in recombinant phytochrome. Eur J Biochem 236:978–983
Hahn J, Strauss HM, Landgraf FT, Gimenez HF, Lochnit G, Schmieder P, Hughes J (2006) Probing protein-chromophore interactions in Cph1 phytochrome by mutagenesis. FEBS J 273:1415–1429
Hübschmann T, Börner T, Hartmann E, Lamparter T (2001a) Characterization of the Cph1 holo-phytochrome from Synechocystis sp PCC 6803. Eur J Biochem 268:2055–2063
Hübschmann T, Jorissen HJMM, Börner T, Gärtner W, deMarsac NT (2001b) Phosphorylation of proteins in the light-dependent signalling pathway of a filamentous cyanobacterium. Eur J Biochem 268:3383–3389
Jorissen HJMM, Quest B, Remberg A, Coursin T, Braslavsky SE, Schaffner K, deMarsac NT, Gärtner W (2002) Two independent, light-sensing two-component systems in a filamentous cyanobacterium. Eur J Biochem 269:2662–2671
Karniol B, Wagner JR, Walker JM, Vierstra RD (2005) Phylogenetic analysis of the phytochrome superfamily reveals distinct microbial subfamilies of photoreceptors. Biochem J 392:103–116
Kufer W, Scheer H (1979) Studies on plant bile pigments, VII, preparation and characterization of phycobiliproteins with chromophores chemically modified by reduction. Hoppe Seylers Z Physiol Chem 360:935–956
Lamparter T, Mittmann F, Gärtner W, Börner T, Hartmann E, Hughes J (1997) Characterization of recombinant phytochrome from the cyanobacterium Synechocystis. Proc Natl Acad Sci USA 94:11792–11797
Mozley D, Remberg A, Gärtner W (1997) Large scale generation of affinity-purified recombinant phytochrome chromopeptide. Photochem Photobiol 66:710–715
Quest B, Gärtner W (2004) Chromophore selectivity in bacterial phytochromes: dissecting the process of chromophore attachment. Eur J Biochem 271:1117–1126
Remberg A, Lindner I, Lamparter T, Hughes J, Kneip C, Hildebrandt P, Braslavsky SE, Gärtner W, Schaffner K (1997) Raman spectroscopic and light-induced kinetic characterization of a recombinant phytochrome of the cyanobacterium Synechocystis. Biochemistry 36:13389–13395
Remberg A, Schmidt P, Braslavsky SE, Gärtner W, Schaffner K (1999) Differential effects of mutations in the chromophore pocket of recombinant phytochrome on chromoprotein assembly and Pr-to-Pfr photoconversion. Eur J Biochem 266:201–208
Schäfer E, Nagy F (eds) (2006) Photomorphogenesis in plants and bacteria. Springer, Dordrecht
Schmidt P, Gensch T, Remberg A, Gärtner W, Braslavsky SE, Schaffner K (1998) The complexity of the Pr → Pfr phototransformation kinetics is an intrinsic property of homogeneous native phytochrome. Photochem Photobiol 68:754–761
Schneider-Poetsch HAW (1992) Signal transduction by phytochrome: phytochromes have a module related to the transmitter modules of bacterial sensor proteins. Photochem Photobiol 56:839–846
Stock JB, Da Re S (2000) Signal transduction: response regulators on and off. Curr Biol 10:R420–R422
van Thor JJ, Borucki B, Crieelard W, Otto H, Lamparter T, Hughes J, Hellingwerf KJ, Heyn MP (2001) Light-induced proton release and proton uptake reactions in the cyanobacterial phytochrome Cph1. Biochemistry 40:11460–11471
Wagner JR, Brunzelle JS, Forest KT, Vierstra RD (2005) A light-sensing knot revealed by the structure of the chromophore-binding domain of phytochrome. Nature 438:325–331
Wurgler-Murphy SM, Saito H (1997) Two-component signal transducers and MAPK cascades. Trends Biochem Sci 22:172–176
Yeh KC, Lagarias JC (1998) Eukaryotic phytochromes: light-regulated serine/threonine protein kinases with histidine kinase ancestry. Proc Natl Acad Sci USA 95:13976–13981
Yeh K-C, Wu S-H, Murphy JT, Lagarias JC (1997) A cyanobacterial phytochrome two-component light sensory system. Science 277:1505–1508
Acknowledgment
Part of this work was supported by a grant from VIBS (virtual institute for biological structure investigation, FZ Jülich, Helmholtz Society).
Author information
Authors and Affiliations
Corresponding author
Additional information
Proceedings of the XVIII Congress of the Italian Society of Pure and Applied Biophysics (SIBPA), Palermo, Sicily, September 2006.
Rights and permissions
About this article
Cite this article
Sharda, S., Shah, R. & Gärtner, W. Domain interaction in cyanobacterial phytochromes as a prerequisite for spectral integrity. Eur Biophys J 36, 815–821 (2007). https://doi.org/10.1007/s00249-007-0171-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00249-007-0171-1