Abstract
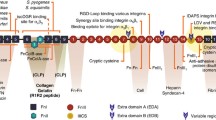
Polymerization of soluble fibronectin molecules results in fibres that are visible as networks using fluorescently labelled fibronectin protomers or by antibody labelling. Displacement of fibres composed of modified protomers in living cells provides information regarding matrix structure, organization, and movement. A static analysis of fibronectin structures and patterns of organization provide insight into their reorganization during adhesion and motility. Confocal microscopy and atomic force microscopy (AFM) reveal fibronectin-containing networks aligned in arrays perpendicular to the retracting cell edge and in apparently disordered networks of fibres under the cell. The change in patterns suggests a reorganization of fibronectin from disordered arrays used for adhesion into ordered arrays during movement of the cell. Comparison of confocal images with corresponding AFM images confirms that the fibres left on the surface as the cell moves away do contain fibronectin. The orientation of these fibres relative to the tail (uropod) and the receding edges of the cell leads us to propose that cells generate a force on the fibres that exceeds the adhesion force of the fibres to the surface causing them to pull fibronectin fibres into straight arrays. However, when the fibres are parallel to the direction of pull, the fibres remain attached to the surface. The data supports the hypothesis that disorganized, linear fibres are the product of Fn polymerization induced by the cell beneath it and serve to adhere the cell to the substrate as the cell spreads, whereas arrays of fibres found outside the cell are formed as existing fibrils and reorganize during cell motility.










Similar content being viewed by others
Abbreviations
- Fn:
-
Fibronectin
- ECM:
-
Extracellular matrix
- AFM:
-
Atomic Force Microscopy
References
Adams JC, Watt FM (1990) Changes in keratinocyte adhesion during terminal differentiation: reduction in fibronectin binding precedes α5β4 integrin loss from the cell surface. Cell 63:425–435
Alberts B, Johnson A, Lewis J, Raff M, Roberts K, Walter P (2002) Molecular biology of the cell, 4th edn. Garland, New York
Anderson EH, Ruegsegger MA, Murugesan G, Kottke-Merchant K, Marchant RE (2004) Extracellular matrix-like surfactant polymers containing arginine–glycine–aspartic acid (RGD) peptides. Macromol Biosci 4(8):766–775
Baneyx G, Vogel V (1999) Self-assembly of fibronectin into fibrillar networks underneath dipalmitoyl phosphatidylcholine monolayers: role of lipid matrix and tensile forces. Proc Natl Acad Sci USA 96(22):12518–12523
Baneyx G, Baugh L, Vogel V (2001) Coexisting conformations of fibronectin in cell culture imaged using fluorescence resonance energy transfer. Proc Natl Acad Sci USA 98(25):14464–14468
Baneyx G, Baugh L, Vogel V (2002) Fibronectin extension and unfolding within cell matrix fibrils controlled by cytoskeletal tension. Proc Natl Acad Sci USA 99(8):5139–5143
Baugh L, Vogel V (2004) Structural changes of fibronectin adsorbed to model surfaces probed by fluorescent resonance energy transfer. J Biomed Mater Res 3:525–534
Bray D (2001) Cell movements from molecules to motility, 2nd edn. Garland, New York
Brockwell DJ, Paci E, Zinober RC, Beddard GS, Olmsted PD, Smith DA, Perham RN, Radford SE (2003) Pulling geometry defines the mechanical resistance of a beta-sheet protein. Nat Struct Biol 10:731–737
Burridge K, Fath K, Kelly T, Nucko G, Turner C (1988) Focal adhesions: transmembrane junctions between the extracellular matrix and cytoskeleton. Annu Rev Cell Biol 4:487–525
Carrion-Vazquez M, Li H, Lu H, Marszalek PE, Oberhauser AF, Fernandez JM (2003) The mechanical stability of ubiquitin is linkage dependent. Nat Struct Biol 10:738–743
Chen WT (1981) Mechanism of retraction of the trailing edge during fibroblast movement. J Cell Biol 90:187–200
Chen CS, Mrksich M, Huang S, Whitesides G, Ingber DE (1997) Geometric control of cell life and death. Science 276:1425–1428
Choquet D, Felsenfeld DP, Sheetz MP (1997) Extracellular matrix rigidity causes strengthening of integrin–cytoskeleton linkages. Cell 88:39–48
Craig D, Gao M, Schulten K, Vogel V (2004) Tuning the mechanical stability of fibronectin type III modules through sequence variations. Structure 12:21–30
Davies PF, Barbee KA, Volin MV, Robotewskyj A, Chen J, Joseph L, Griem ML, Wernick MN, Jacobs E, Polacek DC, DePaola N, Barakat AI (1997) Spatial relationships in early signaling events of flow-mediated endothelial mechanotransduction. Annu Rev Physiol 59:527–549
Dembo M, Wang Y-L (1999) Stresses at the cell-to-substrate interface during locomotion of fibroblasts. Biophys J 76:2307–2316
DiMilla PA, Barbee K, Lauffenburger DA (1991) Mathematical model for the effects of adhesion and mechanics on cell migration speed. Biophys J 60:15–37
Erickson HP (2002) Stretching fibronectin. J Muscle Res Cell Motil 23:575–580
Galbraith CG, Sheetz MP (1998) Forces on adhesion contacts affect cell function. Curr Opin Cell Biol 10:566–571
Gao M, Craig D, Lequin O, Campbell ID, Vogel V, Schulten K (2003) Structure and functional significance of mechanically unfolded fibronectin type III1 intermediates. Proc Natl Acad Sci USA 100(25):14784–14789
Geiger B, Bershadsky A, Pankov R, Yamada KM (2001) Transmembrane crosstalk between the extracellular matrix–cytoskeleton crosstalk. Natl Rev Mol Cell Biol 11:793–805
Henderson E, Haydon PG, Sakaguchi DS (1992) Actin filament dynamics in living glial cells imaged by atomic force microscopy. Science 257:1944–1946
Hynes RO (1990) Fibronectins. Springer, Berlin Heidelberg New York
Hynes RO (1999) Cell adhesion: old and new questions. Trends Cell Biol 9(12):M33–M37
Hynes RO, Destree AT (1978) Relationships between fibronectin (LETS protein) and actin. Cell 15:875–886
Ingber DE (1997) Tensegrity: the architectural basis of cellular mechanotransduction. Annu Rev Physiol 59:575–599
Ingber DE (2003) Tensegrity II. How structural networks influence cellular information processing networks. J Cell Sci 116:1397–1408
Krammer A, Lu H, Isralewitz B, Schulten K, Vogel V (1999) Forced unfolding of the fibronectin type III module reveals a tensile molecular recognition switch. Proc Natl Acad Sci USA 96:1351–1356
Krammer A, Craig D, Thomas WE, Schulten K, Vogel V (2002) A structural model for force regulated integrin binding to fibronectin’s RGD-synergy site. Matrix Biol 21:139–147
Mathur AB, Truskey GA, Reichert WM (2000) Atomic force and total internal reflection fluorescence microscopy for the study of force transmission in endothelial cells. Biophys J 78:1725–1735
Munevar S, Wang Y-L, Dembo M (2001) Traction force microscopy of migrating normal and H-ras transformed 3T3 fibroblasts. Biophys J 80:1744–1757
Ohashi T, Kiehart DP, Erickson HP (1999) Dynamics and elasticity of the fibronectin matrix in living cell culture visualized by fibronectin-green fluorescent protein. Proc Natl Acad Sci USA 96:2153–2158
Ohashi T, Kiehart DP, Erickson HP (2002) Dual labelling of the fibronectin matrix and actin cytoskeleton with green fluorescent protein variants. J Cell Sci 115:1221–1229
Pickering JG, Chow LH, Li S, Rogers KA, Rocnik EF, Zhong R, Chan BMC (2000) alpha5beta1 integrin expression and luminal edge fibronectin matrix assembly by smooth muscle cells after arterial injury. Am J Pathol 156(2):453–465
Potts JR, Campbell ID (1994) Fibronectin structure and assembly. Curr Opin Cell Biol 6:648–655
Renner BL, Jorgensen M, Markowski K, Salchert C, Werner TP (2004) Control of fibronectin displacement on polymer substrates to influence endothelial cell behaviour. J Mater Sci Mater Med 15:387–390
Riveline D, Zamir E, Balaban NQ, Schwarz US, Ishizaki T, Narumiya S, Kam Z, Geiger B, Bershadsky AD (2001) Focal contacts as mechanosensors: externally applied local mechanical force induces growth of focal contacts by an mDia1-dependent and ROCK-independent mechanism. J Cell Biol 153:1175–1186
Schmidt CE, Horwitz AF, Lauffenburger DA, Sheetz MP (1993) Integrin–cytoskeletal interactions in migrating fibroblasts are dynamic, asymmetric, and regulated. J Cell Biol 123:977–991
Shaub A (1999) Unravelling the extracellular matrix. Nat Cell Biol 1:E173–E175
Sheetz MP, Felsenfeld DP, Galbraith CG (1998) Cell migration: regulation of force on extracellular-matrix–integrin complexes. Trends Cell Biol 8:51–54
Wang Y-L (1985) Exchange of actin subunits at the leading edge of living fibroblasts: possible role of treadmilling. J Cell Biol 101:597–602
Wang N, Butler JP, Ingber ED (1993) Mechanotransduction across the cell surface and through the cytoskeleton. Science 260:1124–1127
Zhong CL, Chrzanowska-Wodnicka M, Brown J, Shaub A, Belkin AM, Burridge K (1998) Rho-mediated contractility exposes a cryptic site in fibronectin and induces fibronectin matrix assembly. J Cell Biol 141:539–551
Acknowledgements
The authors would like to thank Dr. Niki Boyd for fruitful discussions, the Petersen and Norton group members, and the Sandig lab in the Department of Anatomy and Cell Biology for their technical assistance. We would also like to acknowledge financial support from NSERC for strategic grants and CFI for infrastructure grants to NOP and PRN as well as OGSST and NSERC for postgraduate scholarships for KLD.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Rights and permissions
About this article
Cite this article
De Jong, K.L., MacLeod, H.C., Norton, P.R. et al. Fibronectin organization under and near cells. Eur Biophys J 35, 695–708 (2006). https://doi.org/10.1007/s00249-006-0081-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00249-006-0081-7