Abstract
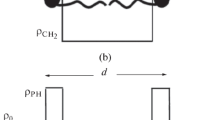
The small-angle neutron scattering (SANS) data of 12 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine (POPC) dispersions at low lipid concentration (1 mg per 100-mg heavy water) prepared by 5, 9 and 29 extrusions through filters of pores with 50, 100, 200 and 400 nm diameter are presented. They were analyzed within a theory that permits the determination of both structural and hydration parameters of the bilayers as well as the portions of multilamellar vesicles in dispersions with negligible long-range order between the vesicles. The scattering length density profile across the bilayers is approximated by assuming a central hydrocarbon core surrounded by a water-accessible coat. It is modeled by two different forms of functions. In the boat model, the scattering length density of the coat changes linearly from core to water, whereas in the strip model it is constant across the water-accessible coat. It was found that the boat model reflects the reality better than the strip model. The decrease of the multilamellar vesicle portions, either with increasing the number of extrusions at same filter size and with decreasing the filter size, was characterized quantitatively.





Similar content being viewed by others
References
Cornell BR, Fletcher GC, Middlehurst J, Separovic F (1981) Temperature dependence of the size of phospholipids vesicles. Biochim Biophys Acta 642:375–380
Engelhaaf SU, Wehrli E, Müller M, Adrian M, Schurtenberger P (1996) Determination of the size distribution of lecithin liposomes: a comparative study using freeze fracture, cryoelectron microscopy and dynamic light scattering. J Microsc 184:214–228
Fromherz P (1983) Lipid-vesicle structure: size control by edge-active agents. Chem Phys Lett 94:259–265
Gabriel NE, Roberts MF (1984) Spontaneous formation of stable unilamellar vesicles. Biochem 23:4011–4015
Gradshtein IS, Ryzhik IM (1971) Tables of integrals (in Russian). Nauka, Moscow
Hallett FR, Nickel B, Samuels C, Krygsman PH (1991) Determination of vesicle size distributions by freeze-fracture electron microscopy. J Electr Microsc Tech 17:459
Hope MJ, Bally MB, Webb G, Cullis PR (1985) Production of large unilamellar vesicles by a rapid extrusion procedure. Characterization of size distribution, trapped volume and ability to maintain a membrane potential. Biochim Biophys Acta 812:55–65
Kiselev MA, Lesieur P, Kisselev AM, Lombardo D, Killiany M, Lesieur S (2001) Sucrose solutions as prospective medium to study the vesicle. J Alloys Compd 328:71–76
Mayer LD, Hope MJ, Cullis PR (1986) Vesicles of variable sizes produced by a rapid extrusion procedure structure: SAXS and SANS study. Biochim Biophys Acta 858:161–168
Nelder JA, Mead R (1965) A simplex method for function minimization. Comput J 7:308
New RRC (1990) Liposomes: a practical approach. IRL Press, Oxford
Pedersen J S, Posselt D, Mortensen K (1990) Analytical treatment of the resolution function for small-angle scattering. J Appl Cryst 23:321–333
Rosta L (2002) Cold neutron research facility at the Budapest Neutron Centre. Appl Phys A74[Supp l.]:S52–S54
Schmiedel H, Jörchel P, Kiselev M, Klose G (2001) determination of structural parameters and hydration of unilamellar POPC/C12E4 vesicles at high water excess from neutron scattering curves using a novel method of evaluation. J Phys Chem B 105:111–117
Acknowledgements
This work has been supported by Bundesministerium für Bildung und Forschung (BMBF grant DUBLEI 03) and the EU, contract HPRI-CT-1999-00099 Budapest.
Author information
Authors and Affiliations
Corresponding author
Appendices
Appendix 1: Derivation of Eq (1)
The neutron coherent scattering amplitude of the M-vesicle depicted in Fig. 1 is given by
where
is the form factor of the m-th bilayer in the vesicle. r m =r 0+m·d is the midpoint radius of that bilayer, and the scattering length density difference between the membrane and the solvent is described by ρ(x) at point x. As the bilayers in Fig. 1 are assumed to be symmetric, i.e.,
we obtain for the integral in (8)
with
and
in case of the strip and the boat model, respectively.
The summation in (7) can be performed using the formula
giving
Here \(r_{{0M}} = r_{0} + (M - 1) \cdot \frac{d}{2}\) is the radius of the vesicle consisting of M bilayers with r 0 as the midpoint radius of the innermost bilayer in the vesicle, and
The average coherent cross section of the M-vesicles is given by the averaged squared absolute value of the scattering amplitude:
The second term in the brackets can be neglected with respect to the first one in case of \(r_{{0M}} \gg d,\) giving (1).
Appendix 2: Averaging over vesicle-radius distribution
Using the integral (Gradshtein and Ryzhik 1971)
the averaging over the radius distribution in (1) can be performed readily giving
with
Rights and permissions
About this article
Cite this article
Schmiedel, H., Almásy, L. & Klose, G. Multilamellarity, structure and hydration of extruded POPC vesicles by SANS. Eur Biophys J 35, 181–189 (2006). https://doi.org/10.1007/s00249-005-0015-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00249-005-0015-9