Abstract
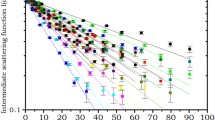
Small angle neutron scattering (SANS) was performed on suspensions of actively metabolising human erythrocytes in the constant shear field induced by a Couette cell. The SANS pattern recorded on a two-dimensional detector was a function of the shear rate; at zero shear, the SANS pattern had radial symmetry around the direction of the beam. The radial average of the SANS pattern consisted of a broad intensity maximum superimposed on a decay. The intensity maximum at q = 0.1 Å-1 was attributed to isotropically oriented self-associated complexes of the tetrameric oxygen transport protein hemoglobin inside the erythrocytes. A flow curve of the cell suspension was used to identify at what shear rate a suspension of uniaxially oriented ellipsoidal cells is produced. The radial symmetry of the SANS patterns persisted until the shear rate was sufficient to produce a suspension of uniaxially oriented ellipsoidal cells. Again, an intensity maximum was present in directions parallel and orthogonal to the shear axis, but this intensity maximum was superimposed upon quite different intensity decays in each direction from that of the primary neutron beam. The angular range of the SANS instrument was limited, however the results from shear-induced structural changes is consistent with a model that involves hemoglobin complexes that are aligned with respect to the plasma membranes of the elongated cells.





Similar content being viewed by others
References
Bateman JB, Hsu SS, Knudsen JP, Yudowitch KL (1953) Hemoglobin spacing in erythrocytes. Arch Biochem Biophys 45:411–422
Bessis M, Mohandas N, Feo C (1980) Automated ektacyometry: a new method of measuring red cell deformability and red cell indices. Blood Cells 6:315–327
Damaschun G, Damaschun H, Gedicke Ch, Müller JJ, Pürschel H-V, Ruckpaul K, Zinke M (1975) Über die supramolekulare Organization des Oxyhämoglobins im Erythrozyten eine Röntgen-kleinwinkelstreuugs-Studie. Acata Biol Med Germ 34:391–398
De Roek RM, Mackely MR (1998) The rheology and micro-structure of equine blood. In: Adams MJ, Mashelkar RA, Pearson JRA, Rennie AR (eds) Dynamics of Complex Fluids. Imperial College Press, The Royal Society, Oxford, pp 338–344
Deuticke B (2003) Membrane lipids as a basis of red cell shape and its alterations. In: Bernhardt I, Ellory JC (eds) Red Cell Membrane Transport in Health and Disease. Springer, Berlin Heidelberg New York, pp 61–80
Edsall JT, Wyman J (1958) Biophysical chemistry, vol 1. Academic Press, New York, pp 537–540
Guinier A, Fournet G (1955) Small-angle Scattering of X-rays. Wiley, New York
Han J, Herzfield J (1993) Macromolecular diffusion in crowded solutions. Biophys J 65:1155–1161
Hayter JB, Penfold J (1981) Self-consistent structural and dynamic study of concentrated micelle solutions. J Chem Soc Faraday Trans I 77:1851–1863
Ise N, Konishi T, Yamanaka J (2001) X-ray scattering study of ionic colloidal crystals. Curr Opin Colloid Interface Sci 6:126–131
Jones CR, Johnson Jr CS, Penniston JT (1978) Photon correlation spectroscopy of oxy-HbA and oxy HbS. Biopolymers 17:1581–1593
Kang I S (2002) A microscopic study on the rheological properties of human blood in the low concentration limit. Korean-Aust J Rheol 14:77–86
Krueger S, Nossal R (1988) SANS studies of interacting hemoglobin in intact erythrocytes. Biophys J 53:97–105
Krueger S, Chen S H, Hofrichter J, Nossal R (1990) Small angle neutron scattering studies of HbA in concentrated solution. Biophys J 55:745–757
Kuchel PW, Chapman BE (1991) Translational diffusion of hemoglobin in human erythrocytes and hemolysates. J Magn Reson 95:574–580
Maher AD, Kuchel PW (2003) The Gàrdos channel: a review of the Ca2+-activated K+ channel in human erythrocytes. Intl J Biochem Cell Biol 35(8):1181–1197
Maruyama H, Suzuki A, Seki H (2000) Adsorption of water-soluble proteins onto bubbles in continuous foam separation. J Colloid Interface Sci 224:76–83
Mazeron P, Muller S, El Azouzi H (1997a) On the intensity reinforcements in the small angle light scattering patterns of erythrocytes under shear. Eur Biophys J 26:247–252
Mazeron P, Muller S, El Azouzi H (1997b) Deformation of erythrocytes under shear: a small-angle light scattering study. Biorheology 34:99–110
Minton AP (1980) Thermodynamic nonideality and the dependence of partition coefficient upon solute concentration in exclusion chromatography: application to self-associating and non-self-associating solutes. Application to hemoglobin. Biophys Chem 12:271–277
Mongin AA, Orlov SN (2001) Mechanisms of cell volume regulation and possible nature of the cell volume sensor. Pathophysiology 8:77–88
Penfold J (1988) Small-angle neutron scattering studies of systems undergoing shear. J Appl Cryst 21:770–776
Salem AJ, Fuller GG (1985) Small angle light scattering as a probe of flow-induced particle orientation. J Colloid Interface Sci 108:149–157
Schelten J, Schlecht P, Schatz W, Mayer A (1972) Neutron small angle scattering of hemoglobin. J Biol Chem 247:5436–5441
Schmid-Schönbein H, Wells R (1969) Fluid drop-like transition of erythrocytes under shear. Science 165:288–291
Stewart IM, Chapman BE, Kirk K, Kuchel PW, Lovric VA, Raftos JE (1986) Intracellular pH in stored erythrocytes: refinement and further characterisation of the 31P-NMR methylphosphonate procedure. Biochim Biophys Acta 885:23–33
Wagner NJ (1998) Rheo-optics. Curr Opin Colloid Interface Sci 3:391–400
Warren B E (1992) X-ray Diffraction. Dover, Mineola
Zimmerman SB, Minton AP (1993) Macromolecular crowding: biochemical, biophysical and physiological consequences. Annu Rev Biophys Biomol Struct 22:27–65
Acknowledgements
The authors acknowledge the support of the Australian Institute of Nuclear Science and Engineering for this work through granting access to the ANSTO SANS instrument. P.W.K. also thanks the Australian Research Council for a Discovery grant. The assistance of Gary Straty and Howard Hanley (NIST) in developing the shear cell is acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Garvey, C.J., Knott, R.B., Drabarek, E. et al. Shear-induced alignment of self-associated hemoglobin in human erythrocytes: small angle neutron scattering studies. Eur Biophys J 33, 589–595 (2004). https://doi.org/10.1007/s00249-004-0408-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00249-004-0408-1