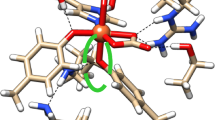
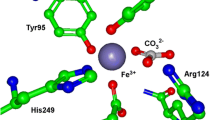
Abstract
Proteins of the transferrin (Tf) family have a role in metal transport in vertebrates and have been extensively studied. The results here reported provide, for the first time, a detailed systematic comparison of metal sites in Tf complexes involving several atoms in the whole protein and in two different types of Tfs. The high interest in the structural variations induced in a metalloprotein upon the uptake of different metals is related to the hypothesis of the metals' involvement in some neuropathologies. We propose a comparative study of the X-ray absorption spectra at the K-edge of iron, copper, zinc and nickel in serotransferrin and ovotransferrin. The experimental data are simulated using an algorithm of the full multiple scattering method. Our results show that: (1) the local structure of each site (N-terminal and C-terminal) is correlated to the ligation state of the other site; (2) the difference between the two proteins is related to site local structure and depends on the metal ion nature being greater in the case of copper and zinc with respect to iron and nickel ions; (3) X-ray spectroscopy is confirmed as a suitable technique able to discriminate between coordination models proposed by X-ray diffraction.













Similar content being viewed by others
Abbreviations
- MS:
-
multiple scattering
- Tf:
-
transferrin
- XANES:
-
X-ray absorption near-edge structure
References
Aisen P (1989) In: Loehr TM (ed) The transferrin iron carriers and iron proteins. VCH, Weinheim, pp 241–371
Aisen P, Leibam A, Zweier J (1978) Stoichiometric and site characteristic of the binding of iron to human transferrin. J Biol Chem 253:1930–1937
Alagna L, Strange RW, Durham P, Hasnain SS (1986) Stereochemisty of copper in superoxide dismutase in solution from X-ray absorption near edge structures (XANES). J Phys (Paris) C8 47:62–73
Anderson BF, Baker HM, Dodson EJ, Norris GE, Rice DW, Baker EN (1989) Structure of human lactoferrin: crystallographic structure analysis and refinement at 2.8 Å resolution. J Mol Biol 209:711–734
Baker EN, Lindley PF (1992) New perspectives on the structure and function of transferrin. J Inorg Biochem 47:147–160
Baldwin DA, De Sousa DMR (1982) The effect of pH on the kinetics of iron release from human transferrin. Biochim Biophys Acta 719:140–146
Bali P, Aisen P (1991) Receptor-modulated iron release from transferrin: differential effects on N- and C-terminal sites. Biochemistry 30:9947–9952
Bayley S, Evans R, Garrat RC (1988) Molecular structure of serum transferrin at 3.3 Å resolution. Biochemistry 27:5804–5812
Bianconi A, Congiu-Castellano A, Durham PF, Hasnain SS, Phillips S (1985) The CO bond angle of carboxymyoglobin determined by angular resolved XANES spectroscopy. Nature 318:685–687
Boffi F, Ascone I, Varoli Piazza A, Girasole M, Della Longa S, Giovannelli A, Congiu Castellano A (2000) Comparative XANES study of serotransferrin and ovotransferrin at Cu K-edge: evidence of interactions among the metal sites. Biometals 13:217–222
Boffi F, Congiu-Castellano A, Varoli-Piazza A, Della Longa S, Girasole M, Yalovega G, Soldatov AV (2001) Iron and copper K-edge XAS study of serotransferrin and ovotransferrin. J Synchrotron Radiat 8:196–198
Brock JH (1989) Iron-binding proteins. Acta Pediatr Scand Suppl 361:31–43
Bruns CM, Nowalk AJ, Arvai AS, McTigue MA, Vaughan KG, Mietzener TA, McRee DE (1997) Structure of Heamophilus influenza Fe3+ binding protein reveals convergent evolution within a superfamily. Nat Struct Biol 4:919–924
Butterworth RM, Gibson JF, Williams J (1975) EPR spectroscopy of iron binding fragments of hen ovotransferrins. Biochem J 149:559–563
Cheung KC, Strange RW, Hasnain SS (2000) 3D EXAFS refinement of the Cu site of structural change at the metal centre in an oxidation-reduction process: an integrated approach combining EXAFS and crystallography. Acta Crystallogr Sect D 56:697–704
Clozza A, Garcia J, Bianconi A, Corma A (1986) Local structure in bifunctional Ni-MoO3 zeolite catalyst by K-Ni EXAFS and XANES spectroscopy. J Phys (Paris) C8 47:313–316
Congiu Castellano A, Castagnola M, Burattini E, Dell'Ariccia M, Della Longa S, Giovannelli A, Durham PJ, Bianconi A (1989) Hetereogenity of the isolated subunits of the fetal and adult human hemoglobin in solution detected by XANES spectroscopy. Biochim Biophys Acta 996:240–246
Congiu Castellano A, Barteri M, Castagnola M, Bianconi A, Borghi E, Della Longa S (1994) Structure-function relationship in the serotransferrin: the role of the pH on the conformational change and the metal ion release. Biochem Biophys Res Commun 198:646–652
Cramer SP (1988) Biochemical application of X-ray absorption spectroscopy. Wiley, New York
Cramer SP (1992) 13-element Ge detector for fluorescence EXAFS. Nucl Instrum Methods A 319:285–289
Crichton RR (1991) Chemistry and biology of the transferrin: inorganic biochemistry of iron metabolism. Horwood, Chichester, pp 101–119
Della Longa S, Soldatov AV, Pompa M, Bianconi A (1995) Atomic and electronic structure probed by X-ray absorption spectroscopy: full multiple scattering analysis with G4 XANES package. Comput Mater Sci 4:199–202
Durham PF (1988) Theory of XANES. In: Prinz R, Koningsberger D (eds) X-ray absorption: principles, applications and techniques of EXAFS, SEXAFS and XAFS. Wiley, New York, pp 53–84
Durham PJ, Pendry JB, Hodges CH (1981) XANES: determination of bond angle and multi-atom correlations in order and disordered systems. Solid State Commun 38:159–161
Evans RW, Crawley JB, Garrat RC, Grossman JG, Neu M, Aitken A, Patel KJ, Meilak A, Wong C, Singh J, Bomford A, Hasnain SS (1994) Characterization and structural analysis of a functional human serum transferrin variant and implications for receptor recognition. Biochemistry 33:12512–12520
Fuggle JC, Inglesfield JE (1992) Unoccupied electronic states. Springer, Berlin Heidelberg New York
Furenlid LR, Renner MW, Fujita E (1995) XAS studies of Ni(I), Ni(II), Ni(III) complexes. Physica B 208:739–742
Garcia J, Bianconi A, Benfatto M, Natoli CR (1986) Coordination geometry of transition metal ions in dilute solutions by XANES. J Phys (Paris) C8 47:49–54
Garrat RC, Evans RE, Hasnain SS, Lindley P (1986) An extended X-ray absorption fine structure investigation of diferric transferrins and their iron-binding fragments. Biochemistry 233:479–484
Garrat RC, Evans R, Hasnain SS, Lindley F (1991) XAFS studies of chicken dicupric ovotransferrin. Biochem J 233:151–155
Grossmann JG, Neu M, Evans RW, Lindley PF, Appel H, Hasnain SS (1993) Metal-induced conformational changes in transferrins. J Mol Biol 229:585–590
Grossmann JG, Crawley JB, Strange RW, Patel KJ, Murphy LM, Neu M, Evans RW, Hasnain SS (1998) The nature of ligand-induced conformational change in transferrin in solution. An investigation using X-ray scattering, XAFS and site-directed mutants. J Mol Biol 279:461–472
Harris WR (1983) Thermodynamic binding constants of the zinc-human serum transferrin complex. Biochemistry 22:3920–3926
Harris WR (1986) Estimation of the ferrous transferrin binding constant based on thermodynamic studies of nickel-transferrin. J Inorg Biochem 27:41–52
Hasnain SS, Hodgson KO (1999) Structure of metal centres in proteins at subatomic resolution. J Synchrotron Radiat 6:852–864
Hirose J, Fujiwara H, Magarifuchi T, Iguti Y, Iwamoto H, Kominami S, Hiromi K (1996) Copper binding selectivity of N and C sites in serum and ovotransferrin. Biochim Biophys Acta 1296:103–111
Kretchmar SA, Raymond KN (1986) Biphasic kinetics and temperature dependence of iron removal from transferrin by 3,4-LICAMS. J Am Chem Soc 108:6212–6218
Kurokawa H, Mikami B, Hirose M (1995) Crystal structure of diferric hen ovotransferrin at 2.4 Å resolution. J Mol Biol 254:196–199
Legrand D, Mazurier J, Montreuil J, Spik G (1988) Structure and spatial conformation of the iron-binding sites of transferrins. Biochimie 70:1185–1195
Lindley PF, Bajaj M, Evans RW, Garrat RC, Hasnain SS (1993) The mechanism of iron uptake by transferrin: the structure of an 18 kDA NII-domain fragment from duck ovotransferrin at 2.3 Å resolution. Acta Crystallogr Sect D 49:292–304
Macgillivray RTA, Moore SA, Chen J, Anderson BF, Baker H, Luo Y, Bewley M, Smith CA, Murphy MEP, Wang Y, Mason AB, Woodworth RC, Brayer GD, Baker EN (1998) Two high-resolution crystal structures of the recombinant N-lobe of human transferrin reveal a structural change implicated in iron release. Biochemistry 37:7919–7928
Mizutani K, Yamashita H, Kurokawa H, Mikami B, Hirose M (1999) Alternative structural state of transferrin. The crystallographic analysis of iron loaded but domain-opened ovotransferrin N-lobe. J Biol Chem 274:10190–10194
Muller JE, Jepsen O, Wilkins JW (1982) X-ray absorption spectra: K-edges of 3d transition metals, L-edges of 3d and 4d metals and M-edges of palladium. Solid State Commun 42:365–368
Natoli CR, Benfatto M, Brouder C, Ruiz Lopez MF, Foulis DL (1990) Multi-channel multiple scattering theory with general potentials. Phys Rev B 42:1944–1968
Roskams AJ, Connor JR (1990) Aluminum access to the brain: a role for transferrin and its receptor. Proc Natl Acad Sci USA 87:9024–9027
Soldatov AV, Ivanchenko TS, Della Longa S, Kotani A, Iwamoto Y, Bianconi A (1994) Crystal-structure effects in the Ce L3-edge X-ray absorption spectrum of CeO2: multiple scattering resonances and many-body final states. Phys Rev B 50:5074–5080
Strange RW, Blackburn NJ, Knowles PF, Hasnain SS (1987) X-ray absorption spectroscopy of metal-histidine coordination in metalloproteins. Exact simulation of the EXAFS of tetrakis(imidazole)copper(II) nitrate and other copper-imidazole complexes by the use of a multiple scattering treatment. J Am Chem Soc 109:7157–7162
Williams J (1975) Iron-binding fragments from the carboxyl-terminal region of hen ovotransferrin. Biochem J 149:237–244
Williams J (1982) The evolution of transferrins. Trends Biochem Sci 7:394–397
Woolery GL, Powers L, Winkler M, Solomon EI, Lerch K, Spiro TO (1984) Extended X-ray absorption fine structure study of the coupled binuclear copper active site of tyrosinase from Neurospora crassa. Biochim Biophys Acta 788:155–161
Zapolski EJ, Princiotto JV (1980) Binding of iron from nitrilotriacetate analogues by human transferrins. Biochemistry 19:3599–3603
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Boffi, F., Ascone, I., Della Longa, S. et al. X-ray absorption near-edge spectroscopy of transferrins: a theoretical and experimental probe of the metal site local structure. Eur Biophys J 32, 329–341 (2003). https://doi.org/10.1007/s00249-003-0283-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00249-003-0283-1