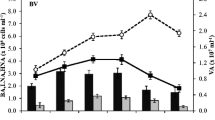
Abstract
Flow cytometric analysis of marine prokaryotes routinely reveals two distinct clusters of heterotrophic cells referred to as high nucleic acid fluorescent (HNA) and low nucleic acid fluorescent (LNA) populations. Evidence suggests that these may represent physiologically and ecologically distinct prokaryote populations. According to the “kill the winner” hypothesis, viral lysis reduces the efficiency of the microbial loop by decreasing the biomass and activity of the most abundant and active members of a population (i.e., competition specialist). Thus, viral-induced mortality may vary according to the physiology of HNA and LNA cells, with implications for the marine carbon cycle. Here, the abundance and production of heterotrophic prokaryotic populations were assessed in the North Atlantic during two phases of the annual plankton cycle and related to bottom-up (i.e., organic carbon variability) and top-down processes (i.e., viral abundance and lytic production). Our results demonstrate that the relative abundance of HNA and LNA heterotrophic cells and heterotrophic prokaryote production vary according to organic carbon variability in the water column, which can be strongly influenced by the physical eddy field (i.e., type of eddy: cyclonic, anticyclonic, or no eddy). In addition, the abundance and lytic production of virus subpopulations were correlated with the cellular production and abundance of heterotrophic HNA and LNA prokaryote communities. Our data suggest group- and activity-specific linkages between hosts and viruses (i.e., HNA-V1 and LNA-V2). Specifically, V1 had a greater contribution to total viral production (i.e., 2.6-fold higher than V2 viruses), similar to their putative host. Finally, we explore potential implications of group- and activity-specific linkages between host and virus groups on the flux of carbon through the microbial food web.







Similar content being viewed by others
References
Zubkov MV, Fuchs BM, Burkill PH, Amann R (2001) Comparison of cellular and biomass specific activities of dominant bacterioplankton groups in stratified waters of the Celtic Sea. Appl Environ Microbiol 67:5210–5218. https://doi.org/10.1128/Aem.67.11.5210-5218.2001
Gasol JM, Del Giorgio PA (2000) Using flow cytometry for counting natural planktonic bacteria and understanding the structure of planktonic bacterial communities. Sci Mar 64:197–224
Li WKW, Jellett JF, Dickie PM (1995) DNA distributions in planktonic bacteria stained with TOTO or TO-PRO. Limnol Oceanogr 40:1485–1495
Button DK, Robertson BR (2001) Determination of DNA content of aquatic bacteria by flow cytometry. Appl Environ Microbiol 67:1636–1645. https://doi.org/10.1128/Aem.67.4.1636-1645.2001
Gasol JM, Zweifel UL, Peters F, Fuhrman JA, Hagstrom A (1999) Significance of size and nucleic acid content heterogeneity as measured by flow cytometry in natural planktonic bacteria. Appl Environ Microbiol 65:4475–4483
Lebaron P, Servais P, Agogue H, Courties C, Joux F (2001) Does the high nucleic acid content of individual bacterial cells allow us to discriminate between active cells and inactive cells in aquatic systems? Appl Environ Microbiol 67:1775–1782. https://doi.org/10.1128/Aem.67.4.1775-1782.2001
Vaque D, Casamayor EO, Gasol JM (2001) Dynamics of whole community bacterial production and grazing losses in seawater incubations as related to the changes in the proportions of bacteria with different DNA content. Aquat Microb Ecol 25:163–177. https://doi.org/10.3354/ame025163
Longnecker K, Sherr BF, Sherr EB (2005) Activity and phylogenetic diversity of bacterial cells with high and low nucleic acid content and electron transport system activity in an upwelling ecosystem. Appl Environ Microbiol 71:7737–7749. https://doi.org/10.1128/Aem.71.12.7737-7749.2005
Jochem FJ, Lavrentyev PJ, First MR (2004) Growth and grazing rates of bacteria groups with different apparent DNA content in the Gulf of Mexico. Mar Biol 145:1213–1225. https://doi.org/10.1007/s00227-004-1406-7
Van Wambeke F, Catala P, Pujo-Pay M, Lebaron P (2011) Vertical and longitudinal gradients in HNA-LNA cell abundances and cytometric characteristics in the Mediterranean Sea. Biogeosciences 8:1853–1863. https://doi.org/10.5194/bg-8-1853-2011
Nishimura Y, Kim C, Nagata T (2005) Vertical and seasonal variations of bacterioplankton subgroups with different nucleic acid contents: possible regulation by phosphorus. Appl Environ Microbiol 71:5828–5836. https://doi.org/10.1128/Aem.71.10.5828-5836.2005
Bouvier T, del Giorgio PA, Gasol JM (2007) A comparative study of the cytometric characteristics of high and low nucleic-acid bacterioplankton cells from different aquatic ecosystems. Environ Microbiol 9:2050–2066. https://doi.org/10.1111/j.1462-2920.2007.01321.x
Sherr EB, Sherr BF, Longnecker K (2006) Distribution of bacterial abundance and cell-specific nucleic acid content in the Northeast Pacific Ocean. Deep-Sea Res Pt I 53:713–725. https://doi.org/10.1016/j.dsr.2006.02.001
Kassen R, Rainey PB (2004) The ecology and genetics of microbial diversity. Annu Rev Microbiol 58:207–231. https://doi.org/10.1146/annurev.micro.58.030603.123654
Pernthaler J (2005) Predation on prokaryotes in the water column and its ecological implications. Nat Rev Microbiol 3:537–546
Fuhrman JA, Noble RT (1995) Viruses and protists cause similar bacterial mortality in coastal seawater. Limnol Oceanogr 40:1236–1242
Sintes E, del Giorgio PA (2014) Feedbacks between protistan single-cell activity and bacterial physiological structure reinforce the predator/prey link in microbial foodwebs. Front Microbiol 5:453. https://doi.org/10.3389/fmicb.2014.00453
Motegi C, Nagata T, Miki T, Weinbauer MG, Legendre L, Rassoulzadegan F (2013) Interactive effects of viral and bacterial production on marine bacterial diversity. PLoS One 8:e76800. https://doi.org/10.1371/journal.pone.0076800
Schwalbach MS, Hewson I, Fuhrman JA (2004) Viral effects on bacterial community composition in marine plankton microcosms. Aquat Microb Ecol 34:117–127. https://doi.org/10.3354/ame034117
Weinbauer MG (2004) Ecology of prokaryotic viruses. FEMS Microbiol Rev 28:127–181
Fuhrman JA, Suttle CA (1993) Viruses in marine planktonic systems. Oceanography 6:51–63
Mojica KDA, Brussaard CPD (2014) Factors affecting virus dynamics and microbial host-virus interactions in marine environments. FEMS Microbiol Ecol 89:495–515. https://doi.org/10.1111/1574-6941.12343
Suttle CA (2005) Viruses in the sea. Nature 437:356–361
Brussaard CPD, Wilhelm SW, Thingstad TF, Weinbauer MG, Bratbak G, Heldal M, Kimmance SA, Middelboe M, Nagasaki K, Paul JH, Schroeder DC, Suttle CA, Vaque D, Wommack KE (2008) Global-scale processes with a nanoscale drive: the role of marine viruses. ISME J 2:575–578
Middelboe M, Jorgensen NOG, Kroer N (1996) Effects of viruses on nutrient turnover and growth efficiency of noninfected marine bacterioplankton. Appl Environ Microbiol 62:1991–1997
Middelboe M, Lyck PG (2002) Regeneration of dissolved organic matter by viral lysis in marine microbial communities. Aquat Microb Ecol 27:187–194
Fuhrman JA (1999) Marine viruses and their biogeochemical and ecological effects. Nature 399:541–548
Prather KA, Bertram TH, Grassian VH, Deane GB, Stokes MD, DeMott PJ, Aluwihare LI, Palenik BP, Azam F, Seinfeld JH, Moffet RC, Molina MJ, Cappa CD, Geiger FM, Roberts GC, Russell LM, Ault AP, Baltrusaitis J, Collins DB, Corrigan CE, Cuadra-Rodriguez LA, Ebben CJ, Forestieri SD, Guasco TL, Hersey SP, Kim MJ, Lambert WF, Modini RL, Mui W, Pedler BE, Ruppel MJ, Ryder OS, Schoepp NG, Sullivan RC, Zhao DF (2013) Bringing the ocean into the laboratory to probe the chemical complexity of sea spray aerosol. Proc Natl Acad Sci U S A 110:7550–7555. https://doi.org/10.1073/pnas.1300262110
Wang XF, Sultana CM, Trueblood J, Hill TCJ, Malfatti F, Lee C, Laskina O, Moore KA, Beall CM, McCluskey CS, Cornwell GC, Zhou YY, Cox JL, Pendergraft MA, Santander MV, Bertram TH, Cappa CD, Azam F, DeMott PJ, Grassian VH, Prather KA (2015) Microbial control of sea spray aerosol composition: a tale of two blooms. ACS Central Sci 1:124–131. https://doi.org/10.1021/acscentsci.5b00148
Behrenfeld MJ, Boss ES (2014) Resurrecting the ecological underpinnings of ocean plankton blooms. Annu Rev Mar Sci 6:167–U208. https://doi.org/10.1146/annurev-marine-052913-021325
Falkowski PG, Ziemann D, Kolber Z, Bienfang PK (1991) Role of eddy pumping in enhancing primary production in the ocean. Nature 352:55–58
McGillicuddy Jr DJ, Robinson AR, Siegel DA, Jannasch HW, Johnson R, Dickey TD, McNeil J, Michaels AF, Knap AH (1998) Influence of mesoscale eddies on new production in the Sargasso Sea. Nature 394:263266
McGillicuddy DJ (2016) Mechanisms of physical-biological-biogeochemical interaction at the oceanic mesoscale. Annu Rev Mar Sci 8:125–159. https://doi.org/10.1146/annurev-marine-010814-015606
Moran XAG, Taupier-Letage I, Vazquez-Dominguez E, Ruiz S, Arin L, Raimbault P, Estrada M (2001) Physical-biological coupling in the Algerian Basin (SW Mediterranean): influence of mesoscale instabilities on the biomass and production of phytoplankton and bacterioplankton. Deep-Sea Res Pt I 48:405–437
Rodriguez J, Tintore J, Allen JT, Blanco JM, Gomis D, Reul A, Ruiz J, Rodriguez V, Echevarria F, Jimenez-Gomez F (2001) Mesoscale vertical motion and the size structure of phytoplankton in the ocean. Nature 410:360–363
Vaillancourt RD, Marra J, Seki MP, Parsons ML, Bidigare RR (2003) Impact of a cyclonic eddy on phytoplankton community structure and photosynthetic competency in the subtropical North Pacific Ocean. Deep-Sea Res Pt I 50:829–847
Lasternas S, Piedeleu M, Sangra P, Duarte CM, Agusti S (2013) Forcing of dissolved organic carbon release by phytoplankton by anticyclonic mesoscale eddies in the subtropical NE Atlantic Ocean. Biogeosciences 10:2129–2143
Baltar F, Aristegui J, Gasol JM, Lekunberri I, Herndl GJ (2010) Mesoscale eddies: hotspots of prokaryotic activity and differential community structure in the ocean. ISME J 4:975–988
Morgan PP (1994) SEAWATER: a library of MATLAB computational routines for the properties of sea water. CSIRO marine laboratories report 222 pg. 29
Behrenfeld MJ, Boss E (2003) The beam attenuation to chlorophyll ratio: an optical index of phytoplankton physiology in the surface ocean? Deep-Sea Res Pt I 50:1537–1549. https://doi.org/10.1016/j.dsr.2003.09.002
Graff JR, Westberry TK, Milligan AJ, Brown MB, Dall'Olmo G, van Dongen-Vogels V, Reifel KM, Behrenfeld MJ (2015) Analytical phytoplankton carbon measurements spanning diverse ecosystems. Deep-Sea Res Pt I 102:16–25. https://doi.org/10.1016/j.dsr.2015.04.006
Parsons TR, Maita Y, Lalli CM (1984) 1.7 - determination of silicate a manual of chemical & biological methods for seawater analysis. Pergamon, Amsterdam, pp 25–28
Grasshoff K (1983) Methods of seawater analysis. Verlag Chemie, Weinheim
Murphy J, Riley JP (1962) A modified single solution method for the determination of phosphate in natural waters. Anal Chim Acta 27:31–36
Carlson CA, Hansell DA, Nelson NB, Siegel DA, Smethie WM, Khatiwala S, Meyers MM, Halewood E (2010) Dissolved organic carbon export and subsequent remineralization in the mesopelagic and bathypelagic realms of the North Atlantic basin. Deep-Sea Res Pt II 57:1433–1445
Smith DC, Azam F (1992) A simple, economical method for measuring bacterial production synthesis rates in seawater. Mar Microbial Food Webs 6:107–114
Simon M, Azam F (1989) Protein content and protein synthesis rates of planktonic marine bacteria. Mar Ecol Prog Ser 51:201–213
Fukuda R, Ogawa H, Nagata T, Koike I (1998) Direct determination of carbon and nitrogen contents of natural bacterial assemblages in marine environments. Appl Environ Microbiol 64:3352–3358
Marie D, Brussaard CPD, Thyrhaug R, Bratbak G, Vaulot D (1999) Enumeration of marine viruses in culture and natural samples by flow cytometry. Appl Environ Microbiol 65:45–52
Mojica KDA, Evans C, Brussaard CPD (2014) Flow cytometric enumeration of marine viral populations at low abundances. Aquat Microb Ecol 71:203–209. https://doi.org/10.3354/ame01672
Brussaard CPD, Marie D, Bratbak G (2000) Flow cytometric detection of viruses. J Virol Methods 85:175–182. https://doi.org/10.1016/S0166-0934(99)00167-6
Brussaard CPD, Payet JP, Winter C, Weinbauer M (2010) Quantification of aquatic viruses by flow cytometry. In: Wilhelm SW, Weinbauer MG, Suttle CA (eds) Manual of aquatic viral ecology. ASLO, pp 102–109
Steward GF, Culley AI, Mueller JA, Wood-Charlson EM, Belcaid M, Poisson G (2013) Are we missing half of the viruses in the ocean? ISME J 7:672–679. https://doi.org/10.1038/ismej.2012.121
Angly FE, Felts B, Breitbart M, Salamon P, Edwards RA, Carlson C, Chan AM, Haynes M, Kelley S, Liu H, Mahaffy JM, Mueller JE, Nulton J, Olson R, Parsons R, Rayhawk S, Suttle CA, Rohwer F (2006) The marine viromes of four oceanic regions. PLoS Biol 4:e368
Wommack KE, Colwell RR (2000) Virioplankton: viruses in aquatic ecosystems. Microbiol Mol Biol Rev 64:69–114
Winget DM, Williamson KE, Helton RR, Wommack KE (2005) Tangential flow diafiltration: an improved technique for estimation of virioplankton production. Aquat Microb Ecol 41:221–232
Paul JH, Weinbauer M (2010) Detection of lysogeny in marine environments. In: Wilhelm SW, Weinbauer MG, Suttle CA (eds) Manual of aquatic viral ecology, pp 30–33
Wilhelm SW, Brigden SM, Suttle CA (2002) A dilution technique for the direct measurement of viral production: a comparison in stratified and tidally mixed coastal waters. Microb Ecol 43:168–173
Parada V, Herndl GJ, Weinbauer MG (2006) Viral burst size of heterotrophic prokaryotes in aquatic systems. J Mar Biol Assoc UK 86:613–621
Development Core Team R (2012) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria
Oksanen J, Blanchet FG, Kindt R, Legendre P, Minchin PR, O'Hara RB, Simpson GL, Solymos P, Stevens MHH, Wagner H (2013) Vegan: community ecology package
Zuur AF, Ieno EN, Elphick CS (2010) A protocol for data exploration to avoid common statistical problems. Methods Ecol Evol 1:3–14
Zuur A, Ieno EN, Walker N, Saveliev AA, Smith GM (2009) Mixed effects models and extensions in ecology with R. Springer, New York
Legendre P, Legendre L (1998) Numerical ecology. Elsevier Science BV, Amsterdam
Jacquet S, Heldal M, Iglesias-Rodriguez D, Larsen A, Wilson W, Bratbak G (2002) Flow cytometric analysis of an Emiliana huxleyi bloom terminated by viral infection. Aquat Microb Ecol 27:111–124
Baudoux AC, Noordeloos AAM, Veldhuis MJW, Brussaard CPD (2006) Virally induced mortality of Phaeocystis globosa during two spring blooms in temperate coastal waters. Aquat Microb Ecol 44:207–217
Mojica KDA, Huisman J, Wilhelm SW, Brussaard CPD (2016) Latitudinal variation in virus-induced mortality of phytoplankton across the North Atlantic Ocean. ISME J 10:500–513
Mary I, Heywood JL, Fuchs BM, Amann R, Tarran GA, Burkill PH, Zubkov MV (2006) SAR11 dominance among metabolically active low nucleic acid bacterioplankton in surface waters along an Atlantic meridional transect. Aquat Microb Ecol 45:107–113. https://doi.org/10.3354/ame045107
Bowman JS, Amaral-Zettler LA, Rich JJ, Luria CM, Ducklow HW (2017) Bacterial community segmentation facilitates the prediction of ecosystem function along the coast of the western Antarctic Peninsula. ISME J 11:1460–1471. https://doi.org/10.1038/ismej.2016.204
Perez MT, Hortnagl P, Sommaruga R (2010) Contrasting ability to take up leucine and thymidine among freshwater bacterial groups: implications for bacterial production measurements. Environ Microbiol 12:74–82. https://doi.org/10.1111/j.1462-2920.2009.02043.x
Schattenhofer M, Wulf J, Kostadinov I, Glockner FO, Zubkov MV, Fuchs BM (2011) Phylogenetic characterisation of picoplanktonic populations with high and low nucleic acid content in the North Atlantic Ocean. Syst Appl Microbiol 34:470–475. https://doi.org/10.1016/j.syapm.2011.01.008
Church MJ, Hutchins DA, Ducklow HW (2000) Limitation of bacterial growth by dissolved organic matter and iron in the Southern Ocean. Appl Environ Microbiol 66:455–466
Carlson CA, Ducklow HW (1996) Growth of bacterioplankton and consumption of dissolved organic carbon in the Sargasso Sea. Aquat Microb Ecol 10:69–85
Kirchman DL (1990) Limitation of bacterial growth by dissolved organic matter in the subarctic Pacific. Mar Ecol Prog Ser 62:47–54
Wear EK, Carlson CA, Windecker LA, Brzezinski MA (2015) Roles of diatom nutrient stress and species identity in determining the short- and long-term bioavailability of diatom exudates to bacterioplankton. Mar Chem 177:335–348
Lopez-Sandoval DC, Rodriguez-Ramos T, Cermeno P, Maranon E (2013) Exudation of organic carbon by marine phytoplankton: dependence on taxon and cell size. Mar Ecol Prog Ser 477:53–60. https://doi.org/10.3354/meps10174
Obernosterer I, Herndl GJ (1995) Phytoplankton extracellular release and bacterial growth: dependence on the inorganic N:P ratio. Mar Ecol Prog Ser 116:247–257
Ewart CS, Meyers MK, Wallner ER, McGillicuddy DJ, Carlson CA (2008) Microbial dynamics in cyclonic and anticyclonic mode-water eddies in the northwestern Sargasso Sea. Deep-Sea Res Pt II 55:1334–1347
Nelson CE, Carlson CA, Ewart CS, Halewood ER (2014) Community differentiation and population enrichment of Sargasso Sea bacterioplankton in the euphotic zone of a mesoscale mode-water eddy. Environ Microbiol 16:871–887
Tarran GA, Zubkov MV, Sleigh MA, Burkill PH, Yallop M (2001) Microbial community structure and standing stocks in the NE Atlantic in June and July of 1996. Deep-Sea Res Pt II 48:963–985
Gaube P, McGillicuddy DJ, Moulin AJ (2019) Mesoscale eddies modulate mixed layer depth globally. https://doi.org/10.1029/2018GL080006
Pedros-Alio C (2012) The rare bacterial biosphere. Annu Rev Mar Sci 4:449–466
Weinbauer MG, Rassoulzadegan F (2004) Are viruses driving microbial diversification and diversity? Environ Microbiol 6:1–11. https://doi.org/10.1046/j.1462-2920.2003.00539.x
Thingstad TF (2000) Elements of a theory for the mechanisms controlling abundance, diversity, and biogeochemical role of lytic bacterial viruses in aquatic systems. Limnol Oceanogr 45:1320–1328
del Giorgio PA, Gasol JM, Vaque D, Mura P, Agusti S, Duarte CM (1996) Bacterioplankton community structure: protists control net production and the proportion of active bacteria in a coastal marine community. Limnol Oceanogr 41:1169–1179
Gonzalez JM, Sherr EB, Sherr BF (1990) Size-selective grazing on bacteria by natural assemblages of estuarine flagellates and ciliates. Appl Environ Microbiol 56:583–589
Baltar F, Palovaara J, Unrein F, Catala P, Horňák K, Simek K, Vaqué D, Maassana R, Gasol JM, Pinhassi J (2016) Marine bacterial community structure resilience to changes in protist predation under phytoplankton bloom conditions. ISME J 10:568–581
Winter C, Bouvier T, Weinbauer MG, Thingstad TF (2010) Trade-offs between competition and defense specialists among unicellular planktonic organisms: the “killing the winner” hypothesis revisited. Microbiol Mol Biol Rev 74:42–57
Azam F, Fenchel T, Field JG, Gray JS, Meyerreil LA, Thingstad F (1983) The ecological role of water-column microbes in the sea. Mar Ecol Prog Ser 10:257–263
Ducklow HW (1999) The bacterial component of the oceanic euphotic zone. FEMS Microbiol Ecol 30:1–10
Legendre L, Lefevre J (1995) Microbial food webs and the export of biogenic carbon in oceans. Aquat Microb Ecol 9:69–77. https://doi.org/10.3354/ame009069
Talarmin A, Van Wambeke F, Catala P, Courties C, Lebaron P (2011) Flow cytometric assessment of specific leucine incorporation in the open Mediterranean. Biogeosciences 8:253–265. https://doi.org/10.5194/bg-8-253-2011
Servais P, Casamayor EO, Courties C, Catala P, Parthuisot N, Lebaron P (2003) Activity and diversity of bacterial cells with high and low nucleic acid content. Aquat Microb Ecol 33:41–51. https://doi.org/10.3354/ame033041
Longnecker K, Sherr BF, Sherr EB (2006) Variation in cell-specific rates of leucine and thymidine incorporation by marine bacteria with high and with low nucleic acid content off the Oregon coast. Aquat Microb Ecol 43:113–125. https://doi.org/10.3354/ame043113
Calvo-Diaz A, Moran XAG (2006) Seasonal dynamics of picoplankton in shelf waters of the southern Bay of Biscay. Aquat Microb Ecol 42:159–174. https://doi.org/10.3354/ame042159
Moran XAG, Bode A, Suarez LA, Nogueira E (2007) Assessing the relevance of nucleic acid content as an indicator of marine bacterial activity. Aquat Microb Ecol 46:141–152. https://doi.org/10.3354/ame046141
Moran XAG, Calvo-Diaz A (2009) Single-cell vs. bulk activity properties of coastal bacterioplankton over an annual cycle in a temperate ecosystem. FEMS Microbiol Ecol 67:43–56. https://doi.org/10.1111/j.1574-6941.2008.00601.x
Azam F (1998) Microbial control of oceanic carbon flux: the plot thickens. Science 280:694–696. https://doi.org/10.1126/science.280.5364.694
Philippot L, Andersson SGE, Battin TJ, Prosser JI, Schimel JP, Whitman WB, Hallin S (2010) The ecological coherence of high bacterial taxonomic ranks. Nat Rev Microbiol 8:523–529. https://doi.org/10.1038/nrmicro2367
Giovannoni SJ, Tripp HJ, Givan S, Podar M, Vergin KL, Baptista D, Bibbs L, Eads J, Richardson TH, Noordewier M, Rappe MS, Short JM, Carrington JC, Mathur EJ (2005) Genomic streamlining in a cosmopolitan oceanic bacterium. Science 309:1241–1245
Wilhelm SW, Suttle CA (1999) Viruses and nutrient cycles in the sea - viruses play critical roles in the structure and function of aquatic food webs. Bioscience 49:781–788
Paul JH (1999) Microbial gene transfer: an ecological perspective. J Mol Microbiol Biotechnol 1:45–50
Clokie MRJ, Shan JY, Bailey S, Jia Y, Krisch HM, West S, Mann NH (2006) Transcription of a ‘photosynthetic’ T4-type phage during infection of a marine cyanobacterium. Environ Microbiol 8:827–835. https://doi.org/10.1111/j.1462-2920.2005.00969.x
Hurwitz BL, Hallam SJ, Sullivan MB (2013) Metabolic reprogramming by viruses in the sunlit and dark ocean. Genome Biol 14:R123. https://doi.org/10.1186/gb-2013-14-11-r123
Anantharaman K, Duhaime MB, Breier JA, Wendt KA, Toner BM, Dick GJ (2014) Sulfur oxidation genes in diverse deep-sea viruses. Science 344:757–760. https://doi.org/10.1126/science.1252229
Bouvier T, Maurice CF (2011) A single-cell analysis of virioplankton adsorption, infection, and intracellular abundance in different bacterioplankton physiologic categories. Microb Ecol 62:669–678
Zhao YL, Temperton B, Thrash JC, Schwalbach MS, Vergin KL, Landry ZC, Ellisman M, Deerinck T, Sullivan MB, Giovannoni SJ (2013) Abundant SAR11 viruses in the ocean. Nature 494:357–360. https://doi.org/10.1038/nature11921
Holmfeldt K, Howard-Varona C, Solonenko N, Sullivan MB (2014) Contrasting genomic patterns and infection strategies of two co-existing Bacteroidetes podovirus genera. Environ Microbiol 16:2501–2513. https://doi.org/10.1111/1462-2920.12391
Suttle CA (2007) Marine viruses - major players in the global ecosystem. Natl Rev 5:801–812
Acknowledgments
We thank the captains and crews of the R/V Atlantis for their help with sampling during the cruises. Furthermore, we thank Nerissa Fisher and Nicholas Huynh for their on-board assistance, Peter Gaube and Ali Della-Penna for their useful discussions regarding the physical data and Luis Bolonas and Steve Giovannoni for sharing microbial genomics data. We further acknowledge NSF-1537943 for C. Carlson.
Funding
This research was supported by the National Aeronautics and Space Administration North Atlantic Aerosol and Marine Ecosystems Study (NAAMES; grant NNX15AF30G).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no competing interests.
Electronic supplementary material
ESM 1
(PDF 694 kb)
Rights and permissions
About this article
Cite this article
Mojica, K.D.A., Carlson, C.A. & Behrenfeld, M.J. Regulation of Low and High Nucleic Acid Fluorescent Heterotrophic Prokaryote Subpopulations and Links to Viral-Induced Mortality Within Natural Prokaryote-Virus Communities. Microb Ecol 79, 213–230 (2020). https://doi.org/10.1007/s00248-019-01393-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00248-019-01393-9