Abstract
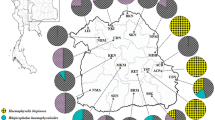
Ixodes ticks transmit infectious agents and also harbor their own parasites and symbionts. The presumptive endosymbiont of Ixodes ricinus, ‘Candidatus Midichloria mitochondrii’, has a unique ability to invade mitochondria within tick ovarian cells and is transovarially transmitted with 100% efficiency. A closely related bacterium, provisionally named Montezuma (now ‘Candidatus Lariskella arthropodarum’), was isolated from the Ixodes persulcatus ticks and human blood in 2004 as well as from Ixodes pavlovskyi in 2015. These microorganisms belong to the family ‘Candidatus Midichloriaceae fam. nov.’ and were detected not only in tick salivary glands, but also in animal blood. Nevertheless, the relative importance of vertical and horizontal routes for their transmission or maintenance in natural tick populations remains unclear. We analyzed the prevalence of L. arthropodarum and M. mitochondrii in two sympatric zones, where I. persulcatus/I. ricinus and I. persulcatus/I. pavlovskyi cohabit and produce interspecific hybrids. A specificity of the associations of L. arthropodarum with I. persulcatus (100%) and M. mitochondrii with I. ricinus (96.2%) was observed in the sympatric zone in Estonia, possibly showing poor contribution of the horizontal route to the overall prevalence of endosymbionts. L. arthropodarum was observed probably multiplying in I. pavlovskyi and also subjected to transovarial transmission, but much less efficiently compared to I. persulcatus. We revealed two new genetic variants of the rrl-rrf intergenic spacer of L. arthropodarum isolated from I. pavlovskyi ticks that possibly could indicate an ongoing process of adaptation of the microorganism to a new host species.


Similar content being viewed by others
References
Sassera D, Beninati T, Bandi C, Bouman EA, Sacchi L, Fabbi M, Lo N (2006) ‘Candidatus Midichloria mitochondrii’, an endosymbiont of the tick Ixodes ricinus with a unique intramitochondrial lifestyle. Int. J. Syst. Evol. Microbiol. 56(Pt 11):2535–2540. doi:10.1099/ijs.0.64386-0
Lewis D (1979) The detection of rickettsia-like microorganisms within the ovaries of female Ixodes ricinus ticks. Z Parasitenkd 59(3):295–298. doi:10.1007/BF00927523
Beninati T, Lo N, Sacchi L, Genchi C, Noda H, Bandi C (2004) A novel alpha-Proteobacterium resides in the mitochondria of ovarian cells of the tick Ixodes ricinus. Appl. Environ. Microbiol. 70(5):2596–2602. doi:10.1128/AEM.70.5.2596-2602.2004
Lo N, Beninati T, Sassera D, Bouman EA, Santagati S, Gern L, Sambri V, Masuzawa T, Gray JS, Jaenson TG, Bouattour A, Kenny MJ, Guner ES, Kharitonenkov IG, Bitam I, Bandi C (2006) Widespread distribution and high prevalence of an alpha-proteobacterial symbiont in the tick Ixodes ricinus. Environ. Microbiol. 8(7):1280–1287. doi:10.1111/j.1462-2920.2006.01024.x
Sassera D, Lo N, Bouman EA, Epis S, Mortarino M, Bandi C (2008) "Candidatus Midichloria" endosymbionts bloom after the blood meal of the host, the hard tick Ixodes ricinus. Appl. Environ. Microbiol. 74(19):6138–6140. doi:10.1128/AEM.00248-08
Epis S, Sassera D, Beninati T, Lo N, Beati L, Piesman J, Rinaldi L, McCoy KD, Torina A, Sacchi L, Clementi E, Genchi M, Magnino S, Bandi C (2008) Midichloria mitochondrii is widespread in hard ticks (Ixodidae) and resides in the mitochondria of phylogenetically diverse species. Parasitology 135(4):485–494. doi:10.1017/S0031182007004052
Beninati T, Riegler M, Vilcins IM, Sacchi L, McFadyen R, Krockenberger M, Bandi C, O’Neill SL, Lo N (2009) Absence of the symbiont Candidatus Midichloria mitochondrii in the mitochondria of the tick Ixodes holocyclus. FEMS Microbiol. Lett. 299(2):241–247. doi:10.1111/j.1574-6968.2009.01757.x
Mariconti M, Epis S, Gaibani P, Dalla Valle C, Sassera D, Tomao P, Fabbi M, Castelli F, Marone P, Sambri V, Bazzocchi C, Bandi C (2012) Humans parasitized by the hard tick Ixodes ricinus are seropositive to Midichloria mitochondrii: is Midichloria a novel pathogen, or just a marker of tick bite? Pathogens and global health 106(7):391–396. doi:10.1179/2047773212Y.0000000050
Bazzocchi C, Mariconti M, Sassera D, Rinaldi L, Martin E, Cringoli G, Urbanelli S, Genchi C, Bandi C, Epis S (2013) Molecular and serological evidence for the circulation of the tick symbiont Midichloria (Rickettsiales: Midichloriaceae) in different mammalian species. Parasit. Vectors 6:350. doi:10.1186/1756-3305-6-350
Serra V, Cafiso A, Bazzocchi C (2016) Molecular and serological evidences of Midichloria mitochondrii transmission to vertebrate hosts during the tick bite. International Journal of Health, Animal Science and Food Safety 3 (1 s). doi:10.13130/2283-3927/7049
Mediannikov O, Ivanov LI, Nishikawa M, Saito R, Sidel’nikov Iu N, Zdanovskaia NI, Mokretsova EV, Tarasevich IV, Suzuki H (2004) Microorganism "Montezuma" of the order Rickettsiales: the potential causative agent of tick-borne disease in the Far East of Russia. Zh. Mikrobiol. Epidemiol. Immunobiol. 1:7–13 [in Russian]
Eremeeva ME, Oliveira A, Robinson JB, Ribakova N, Tokarevich NK, Dasch GA (2006) Prevalence of bacterial agents in Ixodes persulcatus ticks from the Vologda Province of Russia. Ann. N. Y. Acad. Sci. 1078:291–298. doi:10.1196/annals.1374.054
Eremeeva ME, Oliveira A, Moriarity J, Robinson JB, Tokarevich NK, Antyukova LP, Pyanyh VA, Emeljanova ON, Ignatjeva VN, Buzinov R, Pyankova V, Dasch GA (2007) Detection and identification of bacterial agents in Ixodes persulcatus Schulze ticks from the north western region of Russia. Vector Borne Zoonotic Dis 7(3):426–436. doi:10.1089/vbz.2007.0112
Kurilshikov A, Livanova NN, Fomenko NV, Tupikin AE, Rar VA, Kabilov MR, Livanov SG, Tikunova NV (2015) Comparative metagenomic profiling of symbiotic bacterial communities associated with Ixodes persulcatus, Ixodes pavlovskyi and Dermacentor reticulatus ticks. PLoS One 10(7):e0131413. doi:10.1371/journal.pone.0131413
Qiu Y, Nakao R, Ohnuma A, Kawamori F, Sugimoto C (2014) Microbial population analysis of the salivary glands of ticks; a possible strategy for the surveillance of bacterial pathogens. PLoS One 9(8):e103961. doi:10.1371/journal.pone.0103961
Erickson DL, Anderson NE, Cromar LM, Jolley A (2009) Bacterial communities associated with flea vectors of plague. J. Med. Entomol. 46(6):1532–1536
Richard S, Seng P, Parola P, Raoult D, Davoust B, Brouqui P (2009) Detection of a new bacterium related to 'Candidatus Midichloria mitochondrii' in bed bugs. Clin. Microbiol. Infect. 15(Suppl 2):84–85. doi:10.1111/j.1469-0691.2008.02244.x
Matsuura Y, Kikuchi Y, Meng XY, Koga R, Fukatsu T (2012) Novel clade of alphaproteobacterial endosymbionts associated with stinkbugs and other arthropods. Appl. Environ. Microbiol. 78(12):4149–4156. doi:10.1128/AEM.00673-12
Sunagawa S, Woodley CM, Medina M (2010) Threatened corals provide underexplored microbial habitats. PLoS One 5(3):e9554. doi:10.1371/journal.pone.0009554
Vannini C, Ferrantini F, Schleifer KH, Ludwig W, Verni F, Petroni G (2010) "Candidatus Anadelfobacter veles" and "Candidatus Cyrtobacter comes," two new rickettsiales species hosted by the protist ciliate Euplotes harpa (Ciliophora, Spirotrichea). Appl. Environ. Microbiol. 76(12):4047–4054. doi:10.1128/AEM.03105-09
Montagna M, Sassera D, Epis S, Bazzocchi C, Vannini C, Lo N, Sacchi L, Fukatsu T, Petroni G, Bandi C (2013) "Candidatus Midichloriaceae" fam. nov. (Rickettsiales), an ecologically widespread clade of intracellular alphaproteobacteria. Appl. Environ. Microbiol. 79(10):3241–3248. doi:10.1128/AEM.03971-12
Kovalev SY, Mikhaylishcheva MS, Mukhacheva TA (2015) Natural hybridization of the ticks Ixodes persulcatus and Ixodes pavlovskyi in their sympatric populations in Western Siberia. Infect. Genet. Evol. 32:388–395. doi:10.1016/j.meegid.2015.04.003
Kovalev SY, Golovljova IV, Mukhacheva TA (2016) Natural hybridization between Ixodes ricinus and Ixodes persulcatus ticks evidenced by molecular genetics methods. Ticks and tick-borne diseases 7(1):113–118. doi:10.1016/j.ttbdis.2015.09.005
Raychoudhury R, Baldo L, Oliveira DC, Werren JH (2009) Modes of acquisition of Wolbachia: horizontal transfer, hybrid introgression, and codivergence in the Nasonia species complex. Evolution 63(1):165–183. doi:10.1111/j.1558-5646.2008.00533.x
Schuler H, Bertheau C, Egan SP, Feder JL, Riegler M, Schlick-Steiner BC, Steiner FM, Johannesen J, Kern P, Tuba K, Lakatos F, Koppler K, Arthofer W, Stauffer C (2013) Evidence for a recent horizontal transmission and spatial spread of Wolbachia from endemic Rhagoletis cerasi (Diptera: Tephritidae) to invasive Rhagoletis cingulata in Europe. Mol. Ecol. 22(15):4101–4111. doi:10.1111/mec.12362
Reuter M, Pedersen JS, Keller L (2005) Loss of Wolbachia infection during colonisation in the invasive Argentine ant Linepithema humile. Heredity (Edinb) 94(3):364–369. doi:10.1038/sj.hdy.6800601
Schwaiger M, Cassinotti P (2003) Development of a quantitative real-time RT-PCR assay with internal control for the laboratory detection of tick borne encephalitis virus (TBEV) RNA. J. Clin. Virol. 27(2):136–145. doi:10.1016/S1386-6532(02)00168-3
Tamura K, Stecher G, Peterson D, Filipski A, Kumar S (2013) MEGA6: molecular evolutionary genetics analysis version 6.0. Mol. Biol. Evol. 30(12):2725–2729. doi:10.1093/molbev/mst197
Randolph SE, Gern L, Nuttall PA (1996) Co-feeding ticks: epidemiological significance for tick-borne pathogen transmission. Parasitol. Today 12(12):472–479. doi:10.1016/S0169-4758(96)10072-7
Kirstein F, Gray JS (1996) A molecular marker for the identification of the zoonotic reservoirs of Lyme borreliosis by analysis of the blood meal in its European vector Ixodes ricinus. Appl. Environ. Microbiol. 62(11):4060–4065
Moran Cadenas F, Rais O, Humair PF, Douet V, Moret J, Gern L (2007) Identification of host bloodmeal source and Borrelia burgdorferi sensu lato in field-collected Ixodes ricinus ticks in Chaumont (Switzerland). J. Med. Entomol. 44(6):1109–1117. doi:10.1093/jmedent/44.6.1109
Levin ML, Fish D (2000) Immunity reduces reservoir host competence of Peromyscus leucopus for Ehrlichia phagocytophila. Infect. Immun. 68(3):1514–1518. doi:10.1128/IAI.68.3.1514-1518.2000
Karpathy SE, Allerdice ME, Sheth M, Dasch GA, Levin ML (2016) Co-feeding transmission of the Ehrlichia muris-like agent to mice (Mus musculus). Vector Borne Zoonotic Dis 16(3):145–150. doi:10.1089/vbz.2015.1878
Ogden NH, Casey AN, Woldehiwet Z, French NP (2003) Transmission of Anaplasma phagocytophilum to Ixodes ricinus ticks from sheep in the acute and post-acute phases of infection. Infect. Immun. 71(4):2071–2078. doi:10.1128/IAI.71.4.2071-2078.2003
Massung RF, Lee K, Mauel M, Gusa A (2002) Characterization of the rRNA genes of Ehrlichia chaffeensis and Anaplasma phagocytophila. DNA Cell Biol. 21(8):587–596. doi:10.1089/104454902320308960
Vitorino L, Ze-Ze L, Sousa A, Bacellar F, Tenreiro R (2003) rRNA intergenic spacer regions for phylogenetic analysis of Rickettsia species. Ann. N. Y. Acad. Sci. 990:726–733. doi:10.1111/j.1749-6632.2003.tb07451.x
Romanenko V (2011) Long-term dynamics of population density and species composition of pasture ixodid ticks (Parasitiformes, Ixodidae) in anthropogenic and natural areas. Entomol Rev 91(9):1190–1195. doi:10.1134/S0013873811090132
Livanova N, Livanov S, Panov V (2011) Distribution of the ticks Ixodes persulcatus and Ixodes pavlovskyi on the boundary of the forest and forest-steppe zones in the Ob region. Entomol Rev 91(9):1184–1189. doi:10.1134/S0013873811090120
Acknowledgements
We are thankful to Dr. I. Golovljova (Department of Virology, National Institute for Health Development, Tallinn, Estonia) for the material collection and to Dr. K. Chamberlain (Rothamsted Research, UK) for his help in preparing the manuscript. This project was partly supported by Act 211 Government of the Russian Federation, agreement № 02.A03.21.0006, and partly by the Russian Foundation of Basic Research (No. 17-04-00709).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mukhacheva, T.A., Kovalev, S.Y. Bacteria of the Family ‘Candidatus Midichloriaceae’ in Sympatric Zones of Ixodes Ticks: Genetic Evidence for Vertical Transmission. Microb Ecol 74, 185–193 (2017). https://doi.org/10.1007/s00248-017-0932-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00248-017-0932-z