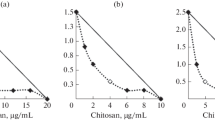
Abstract
We used both aerobic and anaerobic liquid co-cultures, prepared with Luria Bertani broth, to study the effect of bacteria on the survival of Candida albicans in the external environment, away from an animal host. The bacteria were represented by Aeromonas hydrophila, Bacillus cereus, Bacillus subtilis, Clostridium, Enterobacter, Klebsiella pneumoniae, Kluyvera ascorbata and Serratia marcescens. Under aerobic conditions, the yeast’s growth was inhibited in the presence of bacterial growth; however, under anaerobic conditions, yeast and bacterial growth in co-cultures was similar to that observed for pure cultures. Subsequent assays revealed that the majority of bacterial strains aerobically produced extracellular hydrolytic enzymes capable of yeast cell wall hydrolysis, including chitinases and mannan-degrading enzymes. In contrast, except for the A. hydrophila strain, these enzymes were not detected in anaerobic bacterial cultures, nor was the antimicrobial compound prodigiosin found in anaerobic cultures of S. marcescens. When we suspended C. albicans cells in crude extracellular enzyme preparations from K. pneumoniae and S. marcescens, we detected no negative effect on yeast viability. However, we found that these preparations enhance the toxicity of prodigiosin towards the yeast, especially in combination with mannan-degrading enzymes. Analyses of the chitin and mannan content of yeast cell walls revealed that less chitin was produced under anaerobic than aerobic conditions; however, the levels of mannan, known for its low permeability, remained the same. The latter phenomenon, as well as reduced production of the bacterial enzymes and prodigiosin, may contribute to anaerobic growth and survival of C. albicans in the presence of bacteria.






Similar content being viewed by others
References
Aguilar-Uscanga B, Francois JM (2003) A study of the yeast cell wall composition and structure in response to growth conditions and mode of cultivation. Lett Appl Microbiol 37:268–274
Ahmadi K, Yazdi MT, Najafi MF, Shahverdi AR, Faramarzi MA, Zarrini G, Behravan J (2008) Isolation and characterization of a chitionolytic enzyme producing microorganism, Paenibacillus chitinolyticus JK2 from Iran. Research J of Microbiol 3:395–404
Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ (1990) Basic local alignment search tool. J Mol Biol 215:403–410
Atlas RM (1993) Handbook of microbiological media. CRC Press, Boca Raton, FL
Azam F, Malfatti F (2007) Microbial structuring of marine ecosystems. Nature Rev Microbiol 5:782–791
Bao-qin H, Chang-ying YU, Wan-shun LIU, Ji-xun DAI (2004) Purification and inhibition fungal growth of chitinases from Vibrio pacini. Wuhan Univ J Nat Sci 9:973–978
Bernhardt H, Wellmer A, Zimmermann K, Knoke M (1995) Growth of Candida albicans in normal and altered faecal flora in the model of continuous flow culture. Mycoses 38:265–270
Boon C, Deng Y, Wang LH, He Y, Xu JL, Fan Y, Pan SQ, Zhang LH (2008) A novel DSF-like signal from Burkholderia cenocepacia interferes with Candida albicans morphological transition. ISME J 2:27–36
Britz TJ, Sigge GO, Huisamen N, Kikine T, Ackermann A, Lötter M, Lamprecht C, Kidd M (2013) Fluctuations of indicator and index microbes as indication of pollution over three years in the Plankenburg and Eerste Rivers, Western Cape, South Africa. Water SA 39:457–466
Braun PC, Calderone RA (1978) Chitin synthesis in Candida albicans: comparison of yeast and hyphal forms. J of Bacteriol 133:1472–1477
Brzezinska MS, Jankiewicz U, Burkowska A, Walczak M (2014) Chitinolytic microorganisms and their possible application in environmental protection. Curr Microbiol 68:71–81
Buck JD (1977) Comparison of in situ and in vitro survival of Candida albicans in seawater. Microb Ecol 4:291–302
Buckley HR, Van Uben N (1963) The identification of Candida albicans within two hours by the use of an egg white slide preparation. Sabouraudia 2:205–209
Budavari S, O’neil MJ, Smith A, Heckelman PE, Kinneary JF (1996) The merck index: an encyclopedia of chemicals, drug, and biologicals, 12th edn. Merck & Co. Inc, Whitehouse Station, p 1334, 7948
Bulawa CE, Slater M, Cabib E, Au-Young J, Sburlati A, Adair WL, Robbins P (1986) The S. cerevisiae structural gene for chitin synthase is not required for chitin synthesis in vivo. Cell 46:213–225
Cabib E, Bowers B (1971) Chitin and yeast budding. Localization of chitin in yeast bud scars. J Biol Chem 246:152–159
Castro C, Ribas JC, Valdivieso MH, Varona R, del Rey F, Durán A (1995) Papulacandin B resistance in budding and fission yeasts: isolation and characterization of a gene involved in (1,3)b-D-glucan synthesis in Saccharomyces cerevisiae. J Bacteriol 177:5732–5739
Chang S, Sanada M, Johdo O, Ohta S, Nagamatsu Y, Yoshimoto A (2000) High production of prodigiosin by Serratia marcescens grown on ethanol. Biotechnol Lett 22:1761–1765
Chattaway FW, Holmes MR, Barlow AJE (1968) Cell wall composition of the mycelial and blastospore forms of Candida albicans. J of Gen Microbiol 51:367–376
Chester R, Jr C (2011) Yeasts pathogenic to humans. In: Kurtzman CP, Fell JW, Boekhout T (eds) The yeasts, a taxonomic study, 5th edn. Elsevier Science, Amsterdam, The Netherlands, pp 9–19
Cook WL, Schlitzer RL (1981) Isolation of Candida albicans from freshwater and sewage. Appl Environ Microbiol 41:840–842
Cooke HJ, Phaff HJ, Miller MW, Shifrine M, Knapp EP (1960) Yeasts in polluted water and sewage. Mycologia 52:210–230
Cuskin F, Lowe EC, Temple MJ, Zhu Y, Cameron EA, Pudlo NA, Porter NT, Urs K, Thompson AJ, Cartmell A, Rogowski A, Hamilton BS, Chen R, Tolbert TJ, Piens K, Bracke D, Vervecken W, Hakki Z, Speciale G, Munoz-Munoz JL, Day A, Pena MJ, McLean R, Suits MD, Boraston AB, Atherly T, Ziemer CJ, Williams SJ, Davies GJ, Abbott DW, Martens EC, Gilbert HJ (2015) Human gut Bacteroidetes can utilize yeast mannan through a selfish mechanism. Nature 517:165–169
Dahiya N, Tewari R, Tiwari RP, Hoondal GS (2005) Production of an antifungal chitinase from Enterobacter sp. NRG4 and its application in protoplast production. World J Microbiol Biotechnol 21:1611–1616
De Boer W, Folman LB, Summerbell RC, Boddy L (2005) Living in a fungal world: impact of fungi on soil bacterial niche development. FEMS Microbiol 29:795–811
Dhawan S, Kaur J (2007) Microbial mannanases: an overview of production and applications. Crit Rev Biotechnol 27:197–216
Fell JW, Boekhout T, Fonseca A, Scorzetti G, Statzell-Tallman A (2000) Biodiversity and systematics of basidiomycetous yeasts as determined by large-subunit rDNA D1/D2 domain sequence analysis. Int J Syst Evol Microbiol 50:1351–1371
Gacto M, Vicente‐Soler J, Cansado J, Villa TG (2000) Characterization of an extracellular enzyme system produced by Micromonospora chalcea with lytic activity on yeast cells. J Appl Microbiol 88:961–967
Giri AV, Anandkumar N, Muthukumaran G, Pennathur G (2004) A novel medium for the enhanced cell growth and production of prodigiosin from Serratia marcescens isolated from soil. BMC Microbiol 4:11
Gow NAR, van de Veerdonk FL, Brown AJ, Netea MG (2012) Candida albicans morphogenesis and host defence: discriminating invasion from colonization. Nat Rev Microbiol 10:112–122
Gow NAR, Hube B (2012) Importance of the Candida albicans cell wall during commensalism and infection. Curr Opin Microbiol 15:1–7
Guinea J (2014) Global trends in the distribution of Candida species causing candidemia. Clin Microbiol Infect 20(Suppl 6):5–10
Halder SK, Maity CH, Jana A, Das A, Paul T, Mohapatra PKD, Pati BR, Mondal KCH (2013) Proficient biodegradation of shrimp shell waste by Aeromonas hydrophila SBK1 for the concomitant production of antifungal chitinase and antioxidant chitosaccharides. Int Biodet Biodeg 79:88–97
Han MJ, Lee S (2006) The Escherichia coli proteome: past, present, and future prospects. Microbiol Mol Biol Rev 70:362–439
Han Y, Yang B, Zhang F, Miao X, Li Z (2009) Characterization of antifungal chitinase from marine Streptomyces sp. DA11 associated with South China Sea sponge Craniella australiensis. Mar Biotechnol 11:132–140
He G, Shankar RA, Chzhan M, Samouilov A, Kuppusamy P, Zweier JL (1999) Noninvasive measurement of anatomic structure and intraluminal oxygenation in the gastrointestinal tract of living mice with spatial and spectral EPR imaging. Proc Natl Acad Sci U S A 96:4586e4591
Hogan DA, Kolter R (2002) Pseudomonas-Candida interactions: an ecological role for virulence factors. Science 296:2229–2232
Howard SP, Buckley JT (1982) Membrane glycoprotein receptor and hole-forming properties of a cytolytic protein toxin. Biochem 21:1662–1667
Jadhav M, Kagalkar A, Jadhav S, Govindwar S (2011) Isolation, characterization, and antifungal application of a biosurfactant produced by Enterobacter sp. MS16. Eur J Lipid Sci Technol 113:1347–1356
Kennedy MJ, Volz PA, Edwards CA, Yancey RJ (1987) Mechanisms of association of Candida albicans with intestinal mucosa. J Med Microbiol 24:333–341
Khanafari A, Assadi MM, Fakhr FA (2006) Review of prodigiosin, pigmentation in Serratia marcescens. Online J Biol Sci 6:1–13
Landy M, Warren GH, Roseman SB, Colio LG (1948) Bacillomycin: an antibiotic from Bacillus subtilis active against pathogenic fungi. Proc Soc Exp Biol Med 67:539–541
Lee KL, Buckley HR, Campbell CC (1975) An amino acid liquid synthetic medium for the development of mycellal and yeast forms of Candida albicans. Med Mycol 13:148–153
Long HS, Stander MA, Van Wyk BE (2012) Notes on the occurrence and significance of triterpenoids (asiaticoside and related compounds) and caffeoylquinic acids in Centella species. S Afr J Bot 82:53–59
Lorito M, Peterbauer C, Hayes CK, Harman GE (1994) Synergistic interaction between fungal cell wall degrading enzymes and different antifungal compounds enhances inhibition of spore germination. Microbiol 140:623–629
Macagnan D, Romeiro RS, De Souza JT, Pomella AWV (2006) Isolation of actinomycetes and endospore-forming bacteria from the cacao pod surface and their antagonistic activity against the witches’ broom and black pod pathogens. Phytoparasitica 43:122–132
Maruyama Y, Nakajima T, Ichishima E (1994) A 1, 2-α-D-mannosidase from a Bacillus sp.: purification, characterization, and mode of action. Carbohydr Res 251:89–98
Moran G, Coleman D, Sullivan D (2012) An introduction to the medically important Candida species. In: Calderone RA, Clancy CJ (eds) Candida and candidiasis, 2nd edn. ASM Press, Washington, DC, pp 11–25
Nobile CJ, Bruno VM, Richard ML, Davis DA, Mitchell AP (2003) Genetic control of chlamydospore formation in Candida albicans. Microbiol 149:3629–3637
Nucci M, Anaissie E (2001) Revisiting the source of candidemia: skin or gut? Clin infec dis 33:1959–1967
Pappas PG, Rex JH, Lee J, Hamill RJ, Larsen RA, Powderly W, Kauffman CA, Hyslop N, Mangino JE, Chapman S, Horowitz HW, Edwards JE, Dismukes WE (2003) A prospective observational study of candidemia: epidemiology, therapy, and influences on mortality in hospitalized adult and pediatric patients. Clin Infect Dis 37:634–643
Paulse AN, Jackson VA, Khan W (2009) Comparison of microbial contamination at various sites along the Plankenburg- and Diep Rivers, Western Cape, South Africa. Water SA 35:469–478
Peleg AY, Tampakakis E, Fuchs BB, Eliopoulos GM, Moellering RC Jr, Mylonakis E (2008) Prokaryote-eukaryote interactions identified by using Caenorhabditis elegans. Proc Natl Acad Sci U S A 105:14585–14590
Pfaller MA, Diekema DJ (2007) Epidemiology of invasive candidiasis: a persistent public health problem. Clin Microbiol Rev 20:133–163
Prescott LM, Harley JP, Klein DA (2008) Microbiology, 7th edn. McGraw-Hill, New York
Roberts WK, Selitrennikoff CP (1988) Plant and bacterial chitinases differ in antifungal activity. J of Gen Microbiol 134:169–176
Roessner U, Luedemann A, Brust D, Fiehn O, Linke T, Willmitzer L, Fernie AR (2001) Metabolic profiling allows comprehensive phenotyping of genetically or environmentally modified plant systems. Plant Cell 13:11–29
Roongsawang N, Thaniyavarn J, Thaniyavarn S, Kameyama T, Haruki M, Imanaka T, Morikawa M, Kanaya S (2002) Isolation and characterization of a halotolerant Bacillus subtilis BBK-1 which produces three kinds of lipopeptides: bacillomycin L, plipastatin, and surfactin. Extremophiles 6:499–506
Ruiz‐Herrera J, Victoria Elorza M, Valentín E, Sentandreu R (2006) Molecular organization of the cell wall of Candida albicans and its relation to pathogenicity. FEMS Yeast Res 6:14–29
Salmon K, Hung SP, Mekjian K, Baldi P, Hatfield GW, Gunsalus RP (2003) Global gene expression profiling in Escherichia coli K12: the effects of oxygen availability and FNR. J Biol Chem 278:29837–29855
Shapiro RS, Uppuluri P, Zaas AK, Collins C, Senn H, Perfect JR, Heitman J, Cowen LE (2009) Hsp90 orchestrates temperature-dependent Candida albicans morphogenesis via Ras1-PKA signaling. Curr Biol 19:621–629
Shternshis MV, Beljaev AA, Shpatova TV, Duzhak AB, Panfilova ZI (2006) The effect of chitinase on Didymella applanata, the causal agent of raspberry cane spur light. BioControl 51:311–322
Someya N, Nakajima M, Hirayae K, Hibi T, Akutsu K (2001) Synergistic antifungal activity of chitinolytic enzymes and prodigiosin produced by the biocontrol bacterium Serratia marcescens strain B2 against the gray mold pathogen, Botrytis cinerea. J Gen Plant Pathol 67:312–317
Someya N, Nakajima M, Watanabe K, Akutsu K (2005) Synergistic antifungal activity of the culture filtrates of Serratia marcescens strain B2 and chemical fungicides against the sclerotial viability of the rice sheath blight pathogen, Rhizoctonia solani. Biocontrol Sci 10:97–100
Someya N, Tsuchiya K, Yoshida T, Noguchi MT, Akutsu K, Sawada H (2007) Co-inoculation of an antibiotic-producing bacterium and a lytic enzyme-producing bacterium for the biocontrol of tomato wilt caused by Fusarium oxysporum f. sp. lycopersici. Biocontrol Sci 12:1–6
Stone W, Jones B, Wilsenach J, Botha A (2012) External ecological niche for Candida albicans within reducing, oxygen-limited zones of wetlands. Appl Environ Microbiol 78:2443
Swinburne TR, Barr JG, Brown AE (1975) Production of antibiotics by Bacillus subtilis and their effect on fungal colonists of apple leaf scars. Trans Br Mycol Soc 65:211–217
Takegawa K, Miki S, Jikibara T, Iwahara S (1989) Purification and characterization of exo-α-d-mannosidase from a Cellulomonas sp. Biochim Biophys Acta: General Subjects 991:431–437
Tampakakis E, Peleg AY, Mylonakis E (2009) The interaction of Candida albicans with an intestinal pathogen; Salmonella enterica serovar Typhimurium. Eukaryot Cell 8:732–737
Tanaka H, Phaff HJ (1965) Enzymatic hydrolysis of yeast cell walls I. Isolation of wall-decomposing organisms and separation and purification of lytic enzymes. J Bacteriol 89:1570–1580
Vylkova S, Carman AJ, Danhof HA, Collette JR, Zhou H, Lorenz MC (2011) The fungal pathogen Candida albicans autoinduces hyphal morphogenesis by raising extracellular pH. MBio 2:e00055–11
Wang L, He Y, Gao Y, Wu JE, Dong Y, He C, Wang SX, Weng L, Xu J, Tay L, Fang RX, Zhang L (2004) A bacterial cell-cell communication signal with cross-kingdom structural analogues. Mol Microbiol 51:903–912
Weber A, Kogl SA, Jung K (2006) Time-dependent proteome alterations under osmotic stress during aerobic and anaerobic growth in Escherichia coli. J Bacteriol 188:7165–7175
White TJ, Bruns T, Lee S, Taylor J (1990) Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innes MA, Gelfand DH, Sninsky JJ, Whites TJ (eds) PCR protocols. Academic, San Diego, pp 315–322
Williams RP, Gott CL, Qadri SMH, Scott RH (1971) Influence of temperature of incubation and type of growth medium on pigmentation in Serratia marcescens. J Bacteriol 106:438–443
Williamson N, Fineran P, Leeper F, Salmond G (2006) The biosynthesis and regulation of bacterial prodiginines. Nat Rev Microbiol 4:887–899
Wilson D, Mayer F, Hube B (2012) Gene expression during the distinct stages of candidiasis. In: Calderone RA, Clancy CJ (eds) Candida and candidiasis, 2nd edn. ASM Press, Washington, DC, pp 283–298
Yamamoto S, Nagasaki S (1975) Purification and characterization of an exo α-1, 2-mannanase from Flavobacterium dormitator var. glucanolyticae. Agr Biol Chem 39:1981–1989
Acknowledgments
We acknowledge the assistance of Lucky Mokwena and Dr. Marietjie Stander. This work was supported by the Water Research Commission (WRC) and the National Research Foundation (NRF) of South Africa.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Benadé, E., Stone, W., Mouton, M. et al. Binary Interactions of Antagonistic Bacteria with Candida albicans Under Aerobic and Anaerobic Conditions. Microb Ecol 71, 645–659 (2016). https://doi.org/10.1007/s00248-015-0706-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00248-015-0706-4