Abstract
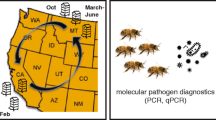
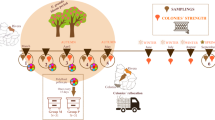
Honeybees are susceptible to a wide range of pathogens, which have been related to the occurrence of colony loss episodes reported mainly in north hemisphere countries. Their ability to resist those infections is compromised if they are malnourished or exposed to pesticides. The aim of the present study was to carry out an epidemiological study in Uruguay, South America, in order to evaluate the dynamics and interaction of honeybee pathogens and evaluate their association with the presence of external stress factors such as restricted pollen diversity and presence of agrochemicals. We monitored 40 colonies in two apiaries over 24 months, regularly quantifying colony strength, parasite and pathogen status, and pollen diversity. Chlorinated pesticides, phosphorus, pyrethroid, fipronil, or sulfas were not found in stored pollen in any colony or season. Varroa destructor was widespread in March (end of summer–beginning of autumn), decreasing after acaricide treatments. Viruses ABPV, DWV, and SBV presented a similar trend, while IAPV and KBV were not detected. Nosema ceranae was detected along the year while Nosema apis was detected only in one sample. Fifteen percent of the colonies died, being associated to high V. destructor mite load in March and high N. ceranae spore loads in September. Although similar results have been reported in north hemisphere countries, this is the first study of these characteristics in Uruguay, highlighting the regional importance. On the other side, colonies with pollen of diverse botanical origins showed reduced viral infection levels, suggesting that an adequate nutrition is important for the development of healthy colonies.




Similar content being viewed by others
References
UNEP (2010) Global honey bee colony disorder and other threats to insect pollinators. UNEP Emerging Issues
Morse RA, Calderone NW (2000) The value of honey bees as pollinators of U.S. crops in 2000. Bee Culture 128:1–15
de Miranda JR, Cordoni G, Budge G (2010) The Acute bee paralysis virus-Kashmir bee virus-Israeli acute paralysis virus complex. J Invertebr Pathol 103(Suppl 1):S30–S47
de Miranda JR, Genersch E (2010) Deformed wing virus. J Invertebr Pathol 103(Suppl 1):S48–S61
Higes M, Martin-Hernandez R, Botias C, Bailon EG, Gonzalez-Porto AV, Barrios L, Del Nozal MJ, Bernal JL, Jimenez JJ, Palencia PG, Meana A (2008) How natural infection by Nosema ceranae causes honeybee colony collapse. Environ Microbiol 10:2659–2669
Higes M, Martin-Hernandez R, Meana A (2010) Nosema ceranae in Europe: an emergent type C nosemosis*. Apidologie 41:375–392
Rosenkranz P, Aumeier P, Ziegelmann B (2010) Biology and control of Varroa destructor. J Invertebr Pathol 103(Suppl 1):S96–S119
Genersch E, Aubert M (2010) Emerging and re-emerging viruses of the honey bee (Apis mellifera L.). Vet Res 41:54
Genersch E (2010) Honey bee pathology: current threats to honey bees and beekeeping. Appl Microbiol Biotechnol 87:87–97
Maori E, Lavi S, Mozes-Koch R, Gantman Y, Peretz Y, Edelbaum O, Tanne E, Sela I (2007) Isolation and characterization of Israeli acute paralysis virus, a dicistrovirus affecting honeybees in Israel: Evidence for diversity due to intra- and inter-species recombination. J Gen Virol 88:3428–3438
Neumann P, Carreck NL (2010) Honey bee colony losses. J Apicult Res 49:1–6
Dainat B, Evans JD, Chen YP, Gauthier L, Neumann P (2012) Predictive markers of honey bee colony collapse. PLoS ONE 7:e32151
Dainat B, Evans JD, Chen YP, Gauthier L, Neumann P (2012) Dead or alive: deformed wing virus and Varroa destructor reduce the life span of winter honeybees. Appl Environ Microbiol 78:981–987
Vanengelsdorp D, Meixner MD (2010) A historical review of managed honey bee populations in Europe and the United States and the factors that may affect them. J Invertebr Pathol 103(Suppl 1):S80–S95
Nazzi F, Brown SP, Annoscia D, Del Piccolo F, Di Prisco G, Varricchio P, Della Vedova G, Cattonaro F, Caprio E, Pennacchio F (2012) Synergistic parasite-pathogen interactions mediated by host immunity can drive the collapse of honeybee colonies. PLoS Pathog 8:e1002735
van Dooremalen C, Gerritsen L, Cornelissen B, van der Steen JJ, van Langevelde F, Blacquiere T (2012) Winter survival of individual honey bees and honey bee colonies depends on level of Varroa destructor infestation. PLoS ONE 7:e36285
Vandame R, Palacio MA (2010) Preserved honey bee health in Latin America: a fragile equilibrium due to low-intensity agriculture and beekeeping? Apidologie 41:243–255
Potts SG, Biesmeijer JC, Kremen C, Neumann P, Schweiger O, Kunin WE (2010) Global pollinator declines: trends, impacts and drivers. Trends Ecol Evol 25:345–353
Naug D (2009) Nutritional stress due to habitat loss may explain recent honeybee colony collapses. Biol Cons 142:2369–2372
Basualdo M, Barragan S, Vanagas L, Garcia C, Solana H, Rodriguez E, Bedascarrasbure E (2013) Conversion of high and low pollen protein diets into protein in worker honey bees (Hymenoptera: Apidae). J Econ Entomol 106:1553–1558
Alaux C, Ducloz F, Crauser D, Le Conte Y (2010) Diet effects on honeybee immunocompetence. Biol Lett 6:562–565
Basualdo M, Barragan S, Antunez K (2014) Bee bread increases honeybee haemolymph protein and promote better survival despite of causing higher Nosema ceranae abundance in honeybees. Environ Microbiol Rep 6:396–400
Huang Z (2012) Pollen nutrition affects honey bee stress resistance. Terr Arthropod Rev 5:175–189
Brodschneider R, Crailsheim K (2010) Nutrition and health in honey bees. Apidologie 41:278–294
Corby-Harris V, Jones BM, Walton A, Schwan MR, Anderson KE (2014) Transcriptional markers of sub-optimal nutrition in developing Apis mellifera nurse workers. BMC Genomics 15:134
van Dooremalen C, Stam E, Gerritsen L, Cornelissen B, van der Steen J, van Langevelde F, Blacquiere T (2013) Interactive effect of reduced pollen availability and Varroa destructor infestation limits growth and protein content of young honey bees. J Insect Physiol 59:487–493
Di Pasquale G, Salignon M, Le Conte Y, Belzunces LP, Decourtye A, Kretzschmar A, Suchail S, Brunet JL, Alaux C (2013) Influence of pollen nutrition on honey bee health: do pollen quality and diversity matter? PLoS ONE 8:e72016
Rinderer TE, Elliott KD (1977) Worker honey bee response to infection with Nosema apis. J Econ Entomol 70:431–433
Foley K, Fazio G, Jensen AB, Hughes WO (2012) Nutritional limitation and resistance to opportunistic Aspergillus parasites in honey bee larvae. J Invertebr Pathol 111:68–73
Rinderer TE, Rothenbuhler WC, Gochnauer TA (1974) The influence of pollen on the susceptibility of honey-bee larvae to Bacillus larvae. J Invertebr Pathol 23:347–350
DeGrandi-Hoffman G, Chen Y, Huang E, Huang MH (2010) The effect of diet on protein concentration, hypopharyngeal gland development and virus load in worker honey bees (Apis mellifera L.). J Insect Physiol 56:1184–1191
Morais M, Moreira L, Feas X, Estevinho LM (2011) Honeybee-collected pollen from five Portuguese Natural Parks: palynological origin, phenolic content, antioxidant properties and antimicrobial activity. Food Chem Toxicol 49:1096–1101
Pascoal A, Rodrigues S, Teixeira A, Feas X, Estevinho LM (2014) Biological activities of commercial bee pollens: antimicrobial, antimutagenic, antioxidant and anti-inflammatory. Food Chem Toxicol 63:233–239
Delaplane KS, Van Der Steen J, Guzman E (2013) Standard methods for estimating strength parameters of Apis mellifera colonies. In V Dietemann; J D Ellis; P Neumann (Eds) The COLOSS BEEBOOK, Volume I: standard methods for Apis mellifera research. J Apicult Res 52(1). doi:10.3896/IBRA.1.52.1.03
Chen Y, Evans J, Hamilton M, Feldlaufer M (2007) The influence of RNA integrity on the detection of honey bee viruses: molecular assessment of different sample storage methods. J Apicult Res 46:81–87
MGAP (2010) Decreto presidencial 14-6-2010. Oxitetraciclina y Fumagilina- Retiro y/o limitación uso en Uruguay. Available at: www.mgap.gub.uy
Rodríguez M, Vargas M, Gerding M, Navarro H, Antúnez K (2012) Viral infection and Nosema ceranae in honey bees (Apis mellifera) in Chile. J Apicult Res 51:285–287
Johnson RM, Evans JD, Robinson GE, Berenbaum MR (2009) Changes in transcript abundance relating to colony collapse disorder in honey bees (Apis mellifera). Proc Natl Acad Sci U S A 106:14790–14795
Kukielka D, Esperon F, Higes M, Sanchez-Vizcaino JM (2008) A sensitive one-step real-time RT-PCR method for detection of deformed wing virus and black queen cell virus in honeybee Apis mellifera. J Virol Methods 147:275–281
Blanchard P, Olivier V, Iscache AL, Celle O, Schurr F, Lallemand P, Ribiere M (2008) Improvement of RT-PCR detection of chronic bee paralysis virus (CBPV) required by the description of genomic variability in French CBPV isolates. J Invertebr Pathol 97:182–185
Palacios G, Hui J, Quan PL, Kalkstein A, Honkavuori KS, Bussetti AV, Conlan S, Evans J, Chen YP, vanEngelsdorp D, Efrat H, Pettis J, Cox-Foster D, Holmes EC, Briese T, Lipkin WI (2008) Genetic analysis of Israel acute paralysis virus: distinct clusters are circulating in the United States. J Virol 82:6209–6217
Yang X, Cox-Foster DL (2005) Impact of an ectoparasite on the immunity and pathology of an invertebrate: evidence for host immunosuppression and viral amplification. Proc Natl Acad Sci U S A 102:7470–7475
Antúnez K, D'Alessandro B, Piccini C, Corbella E, Zunino P (2004) Paenibacillus larvae larvae spores in honey samples from Uruguay: a nationwide survey. J Invertebr Pathol 86:56–58
De Graaf DC, Alippi AM, Antúnez K, Aronstein KA, Budge G, De Koker D, De Smet L, Dingman DW, Evans JD, Foster LJ, Funfhaus A, Garcia-Gonzalez E, Gregorc A, Human H, Murray KD, Nguyen BK, Poppinga L, Spivak M, vanEngelsdorp D, Wilkins S, Genersch E (2013) Standard methods for American foulbrood research. In V Dietemann; J D Ellis; P Neumann (Eds) The COLOSS BEEBOOK, Volume II: standard methods for Apis mellifera pest and pathogen research J Apicult Res 52(1). doi:10.3896/IBRA152111
Genersch E, Forsgren E, Pentikainen J, Ashiralieva A, Rauch S, Kilwinski J, Fries I (2006) Reclassification of Paenibacillus larvae subsp. pulvifaciens and Paenibacillus larvae subsp. larvae as Paenibacillus larvae without subspecies differentiation. Int J Syst Evol Microbiol 56:501–511
Antunez K, Piccini C, Castro-Sowinski S, Rosado AS, Seldin L, Zunino P (2007) Phenotypic and genotypic characterization of Paenibacillus larvae isolates. Vet Microbiol 124:178–183
Fries I, Chauzat MP, Chen YP, Doublet V, Genersch E, Gisder S, Higes M, Mcmahon DP, Martín-Hernández R, Natsopoulou M., Paxton RJ, Tanner G, Webster TC, Williams GR (2013) Standard methods for nosema research. In V Dietemann; J D Ellis, P Neumann (Eds) The COLOSS BEEBOOK: Volume II: Standard methods for Apis mellifera pest and pathogen research. . J Apicult Res 51(5). doi: http://dx.doi.org/10.3896/IBRA.1.52.1.14
Martin-Hernandez R, Meana A, Prieto L, Salvador AM, Garrido-Bailon E, Higes M (2007) Outcome of colonization of Apis mellifera by Nosema ceranae. Appl Environ Microbiol 73:6331–6338
OIE (2008) Chapter 2.2.4. Nosemosis of honeybee. In Manual of Standards for Diagnostic Test and Vaccines [WWW Document] URL http://www.oie.int/fileadmin/Home/esp/Health_standards/tahm/2.02.04_NOSEMOSIS_FINAL.pdf
Dietemann V, Nazzi F, Martin SJ, Anderson D, Locke B, Delaplane KS, Wauquiez Q, Tannahill C, Frey E, Ziegelmann B, Rosenkranz P, Ellis JD (2013) Standard methods for varroa research. In V Dietemann; J D Ellis; P Neumann (Eds). The COLOSS BEEBOOK, Volume II: standard methods for Apis mellifera pest and pathogen research. J Apicult Res 52. doi:10.3896/IBRA.1.52.1.09
Faegri K, Iversen J (1975) Textbook of modern pollen analysis. T Munksgaard Copenhagen: pp.423
Lieux HM (1972) A melissopalynological study of 54 Louisiana (USA) honeys. Rev Palaeobot Palynol 13:95–124
Food Safety and Inspection Service USDoA (2014) Analytical Chemistry Laboratory Guidebook, Residue Chemistry CHC2
Association of Official Analytical Chemists (1990) AOAC, Official Methods of Analysis. 15 th Ed. Methods 983.21 and 968.24 1
Di Prisco G, Pennacchio F, Caprio E, Boncristiani HF Jr, Evans JD, Chen Y (2011) Varroa destructor is an effective vector of Israeli acute paralysis virus in the honeybee, Apis mellifera. J Gen Virol 92:151–155
Martin SJ, Highfield AC, Brettell L, Villalobos EM, Budge GE, Powell M, Nikaido S, Schroeder DC (2012) Global honey bee viral landscape altered by a parasitic mite. Science 336:1304–1306
Antunez K, D'Alessandro B, Corbella E, Zunino P (2005) Detection of chronic bee paralysis virus and acute bee paralysis virus in Uruguayan honeybees. J Invertebr Pathol 90:69–72
Antunez K, D'Alessandro B, Corbella E, Ramallo G, Zunino P (2006) Honeybee viruses in Uruguay. J Invertebr Pathol 93:67–70
Maori E, Paldi N, Shafir S, Kalev H, Tsur E, Glick E, Sela I (2009) IAPV, a bee-affecting virus associated with Colony Collapse Disorder can be silenced by dsRNA ingestion. Insect Mol Biol 18:55–60
Cox-Foster DL, Conlan S, Holmes EC, Palacios G, Evans JD, Moran NA, Quan PL, Briese T, Hornig M, Geiser DM, Martinson V, vanEngelsdorp D, Kalkstein AL, Drysdale A, Hui J, Zhai J, Cui L, Hutchison SK, Simons JF, Egholm M, Pettis JS, Lipkin WI (2007) A metagenomic survey of microbes in honey bee colony collapse disorder. Science 318:283–287
Invernizzi C, Abud C, Tomasco IH, Harriet J, Ramallo G, Campa J, Katz H, Gardiol G, Mendoza Y (2009) Presence of Nosema ceranae in honeybees (Apis mellifera) in Uruguay. J Invertebr Pathol 101:150–153
Costa C, Tanner G, Lodesani M, Maistrello L, Neumann P (2011) Negative correlation between Nosema ceranae spore loads and deformed wing virus infection levels in adult honey bee workers. J Invertebr Pathol 108:224–225
Higes M, Garcia-Palencia P, Martin-Hernandez R, Meana A (2007) Experimental infection of Apis mellifera honeybees with Nosema ceranae (Microsporidia). J Invertebr Pathol 94:211–217
Boncristiani HF Jr, Di Prisco G, Pettis JS, Hamilton M, Chen YP (2009) Molecular approaches to the analysis of deformed wing virus replication and pathogenesis in the honey bee, Apis mellifera. Virol J 6:221
Antunez K, Anido M, Branchiccela B, Harriet J, Campa J, Zunino P (2012) American Foulbrood in Uruguay: twelve years from its first report. J Invertebr Pathol 110:129–131
Cornman RS, Tarpy DR, Chen Y, Jeffreys L, Lopez D, Pettis JS, vanEngelsdorp D, Evans JD (2012) Pathogen webs in collapsing honey bee colonies. PLoS ONE 7:e43562
vanEngelsdorp D, Tarpy DR, Lengerich EJ, Pettis JS (2013) Idiopathic brood disease syndrome and queen events as precursors of colony mortality in migratory beekeeping operations in the eastern United States. Prev Vet Med 108:225–233
Invernizzi C, Santos E, García E, Daners G, Di Landro R, Saadoun A, Cabrera C (2011) Sanitary and nutritional characterization of honeybee colonies in Eucaliptus grandis plantations. Arch Zootec 60:1303–1314
de Oliveira RN, Rehder VL, Oliveira AS, Jeraldo Vde L, Linhares AX, Allegretti SM (2014) Anthelmintic activity in vitro and in vivo of Baccharis trimera (Less) DC against immature and adult worms of Schistosoma mansoni. Exp Parasitol 139:63–72
de Oliveira CB, Comunello LN, Maciel ES, Giubel SR, Bruno AN, Chiela EC, Lenz G, Gnoatto SC, Buffon A, Gosmann G (2013) The inhibitory effects of phenolic and terpenoid compounds from Baccharis trimera in Siha cells: differences in their activity and mechanism of action. Molecules 18:11022–11032
Visintini Jaime MF, Redko F, Muschietti LV, Campos RH, Martino VS, Cavallaro LV (2013) In vitro antiviral activity of plant extracts from Asteraceae medicinal plants. Virol J 10:245
Sulsen V, Guida C, Coussio J, Paveto C, Muschietti L, Martino V (2006) In vitro evaluation of trypanocidal activity in plants used in Argentine traditional medicine. Parasitol Res 98:370–374
Kotwal GJ, Kaczmarek JN, Leivers S, Ghebremariam YT, Kulkarni AP, Bauer G, De Beer C, Preiser W, Mohamed AR (2005) Anti-HIV, anti-poxvirus, and anti-SARS activity of a nontoxic, acidic plant extract from the Trifollium species Secomet-V/anti-vac suggests that it contains a novel broad-spectrum antiviral. Ann N Y Acad Sci 1056:293–302
Çölgeçen H, Koca U, Büyükkartal HN (2011) Use of red clover (Trifolium pratense L.) seeds in human therapeutics. In: Preedy, RV, Ross Watson, R., Vinood, P. (eds) Nuts and deeds in health and disease prevention. Elsevier Inc. pp. 975–980
Alaux C, Dantec C, Parrinello H, Le Conte Y (2011) Nutrigenomics in honey bees: digital gene expression analysis of pollen's nutritive effects on healthy and varroa-parasitized bees. BMC Genomics 12:496
Pareja L, Colazzo M, Perez-Parada A, Niell S, Carrasco-Letelier L, Besil N, Cesio MV, Heinzen H (2011) Detection of pesticides in active and depopulated beehives in Uruguay. Int J Environ Res Public Health 8:3844–3858
Acknowledgments
This work was financed by the Instituto Nacional de Investigación Agropecuaria (INIA), grant INIA-FPTA 258. Authors thank Dennis van Engelsdorp for the help with manuscript organization.
Author information
Authors and Affiliations
Corresponding author
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
SI Figure 1
Seasonal dynamics of adult bee population (number of covered frames, total 440 cm2 per comb), brood area (quarters of comb sides, total 220 cm2 per side of comb) and honey reserves in Apiary 1 and 2, during 2009 and 2010. (GIF 318 kb)
SI Figure 2
Climatic conditions (temperature, relative humidity, pressure and precipitations) in Apiary 1 and 2, during 2009 and 2010. (GIF 96 kb)
SI Figure 3
Seasonal dynamics of the infection rate per apiary with ABPV, BQCV, DWV and SBV in Apiary 1 and 2, during 2009 and 2010. (GIF 313 kb)
ESM 4
(PDF 169 kb)
ESM 5
(PDF 267 kb)
Rights and permissions
About this article
Cite this article
Antúnez, K., Anido, M., Branchiccela, B. et al. Seasonal Variation of Honeybee Pathogens and its Association with Pollen Diversity in Uruguay. Microb Ecol 70, 522–533 (2015). https://doi.org/10.1007/s00248-015-0594-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00248-015-0594-7