Abstract
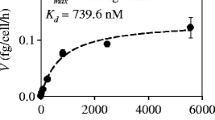
In this study, the mixture of mono- and di-rhamnolipids produced by Pseudomonas aeruginosa DS10-129 was characterized for its toxicity and modulatory effects on Cd availability to different bacteria. Gram-negative naturally bioluminescent Vibrio fischeri and recombinant bioluminescent Pseudomonas fluorescens, P. aeruginosa, Escherichia coli, and Gram-positive Bacillus subtilis were used as model organisms. Rhamnolipids reduced the bioluminescence of these bacteria in less than a second of exposure even in relatively low concentrations (30-min EC50 45–167 mg l−1). Toxicity of Cd to Gram-negative bacteria (30-min EC50 values 0.16 mg l−1 for E. coli, 0.96 mg l−1 for P. fluorescens, and 4.4 mg l−1 for V. fischeri) was remarkably (up to 10-fold) reduced in the presence of 50 mg l−1 rhamnolipids. Interestingly, the toxicity of Cd to Gram-positive B. subtilis (30-min EC50 value 0.49 mg l−1) was not affected by rhamnolipids. Rhamnolipids had an effect on desorption of Cd from soil: 40 mg l−1 rhamnolipids increased the water-extracted fraction of Cd twice compared with untreated control. However, this additionally desorbed fraction of Cd remained bound with rhamnolipids and was not available to bacteria. Hence, in carefully chosen concentrations (still effectively complexing heavy metals but not yet toxic to soil bacteria), rhamnolipids could be applied in remediation of polluted areas.





Similar content being viewed by others
References
Al-Tahhan R, Sandrin TR, Bodour AA, Maier RM (2000) Rhamnolipid-induced removal of lipopolysaccharide from Pseudomonas aeruginosa: effect on cell surface properties and interaction with hydrophobic substrates. Appl Environ Microbiol 66:3262–3268
Ausubel FM, Brent R, Kingston RE, Moore DD, Seidman JG, Smith JA, Strahl K (1987) Current protocols in molecular biology. Wiley, New York
Benincasa M, Abalos A, Oliveira I, Manresa A (2004) Chemical structure, surface properties and biological activities of the biosurfactant produced by Pseudomonas aeruginosa LBI from soap stock. Antonie Van Leeuwenhoek 1:1–8
Bondarenko O, Rõlova T, Kahru A, Ivask A (2008) Bioavailability of Cd, Zn and Hg in soil to nine recombinant luminescent metal sensor bacteria. Sensors 8(11):6899–6923
Bulich AA (1982) A practical and reliable method for monitoring the toxicity of aquatic samples. Process Biochem 17:45–47
Chandrasekaran EV, Bemiller JN (1980) Constituent analyses of glycosaminoglycans. Methods in carbohydrate chemistry. Academic, New York, pp 89–96
Guo YP, Hu YY, Gu RR, Lin H (2009) Characterization and micellization of rhamnolipidic fractions and crude extracts produced by Pseudomonas aeruginosa mutant MIG-N146. J Colloid Interface Sci 331(2):356–363
Haba E, Pinazo A, Jauregui O, Espuny MJ, Infante MR, Manresa A (2003) Physicochemical characterization and antimicrobial properties of rhamnolipids produced by Pseudomonas aeruginosa 47 T2 NCBIM 40044. Biotechnol Bioeng 81(3):316–22
Helander IM, Mattila-Sandholm T (2000) Fluorometric assessment of Gram-negative bacterial permeabilization. J Appl Microbiol 88:213–219
Higgins DG, Sharp PM (1988) CLUSTAL: a package for performing multiple sequence alignment on a microcomputer. Gene 73:237–244
Ivask A, Francois M, Kahru A, Dubourguier HC, Virta M, Douay F (2004) Recombinant luminescent bacterial sensors for the measurement of bioavailability of cadmium and lead in soils polluted by metal smelters. Chemosphere 22:147–156
Ivask A, Rõlova T, Kahru A (2009) A suite of recombinant luminescent bacterial strains for the quantification of bioavailable heavy metals and toxicity testing. BMC Biotech 9:41. doi:10.1186/1472-6710-9-41
Ivask A, Virta M, Kahru A (2002) Construction and use of specific luminescent recombinant bacterial sensors for the assessment of bioavailable fraction of cadmium, zinc, mercury and chromium in the soil. Soil Biol Biochem 34(10):1439–1447
Juwarkar A, Anupa N, Dubey KV, Singh SK, Sukumar D (2007) Biosurfactant technology for remediation of cadmium and lead contaminated soils. Chemosphere 68(10):1996–2002
Kahru A (1993) In vitro toxicity testing using marine luminescent bacteria Photobacterium phosphoreum: the Biotox™ test. ATLA 21(2):210–215
Kahru A, Ivask A, Kasemets K, Põllumaa L, Kurvet I, François M, Dubourguier HC (2005) Biotests and biosensors in ecotoxicological risk assessment of field soils polluted with zinc, lead and cadmium. Environ Toxicol Chem 24(11):2973–2982
Kim IS, Park JS, Kim KW (2001) Enhanced biodegradation of polycyclic aromatic hydrocarbons using nonionic surfactants in soil slurry. Appl Geochem 16:1419–1428
Kimura M (1980) A simple method for estimating evolutionary rate of base substitutions through comparative studies of nucleotide sequences. J Mol Evol 16:111–120
Leedjärv A, Ivask A, Virta M, Kahru A (2006) Analysis of bioavailable phenols from natural samples by recombinant luminescent bacterial sensors. Chemosphere 64:1910–1919
Maslin PM, Maier RM (2000) Rhamnolipid enhanced mineralization of phenanthrene in organic metal co-contaminated soils. Bioremed J 4:295–308
Matsufuji M, Nakata K, Yoshimoto A (1997) High production of rhamnolipids by Pseudomonas aeruginosa growing on ethanol. Biotechnol Lett 19:1213–1215
Meighen EA (1991) Molecular biology of bacterial bioluminescence. Microbiol Rev 55:123–142
Mortimer M, Kasemets K, Heinlaan M, Kurvet I, Kahru A (2008) High throughput kinetic Vibrio fischeri bioluminescence inhibition assay for study of toxic effects of nanoparticles. Toxicol Vitro 22(5):1412–1417
Mulligan CN, Yong RN, Gibbs BF (2001) Heavy metal removal from sediments by biosurfactants. J Hazard Mater 85:111–125
Nitschke M, Costa SGVAO (2007) Biosurfactants in food industry. Trends Food Sci Technol 18(5):252–259
Ochoa-Loza FJ, Artiola JF, Maier RM (2001) Stability constants for the complexation of various metals with a rhamnolipid biosurfactant. J Environ Qual 30(2):479–485
Pornsunthorntawee O, Wongpanit P, Chavadej S, Abe M, Rujiravanit R (2008) Structural and physicochemical characterization of crude biosurfactant produced by Pseudomonas aeruginosa SP4 isolated from petroleum-contaminated soil. Bioresour Technol 99:1589–1595
Rahman KSM, Rahman TJ, Lakshmanaperumalsamy P, Marchant R, Banat IM (2002) Emulsification potential of bacterial isolates with a range of hydrocarbon substrates. Acta Biotechnol 23:335–345
Rahman KSM, Rahman TJ, McClean S, Marchant R, Banat IM (2002) Rhamnolipid biosurfactants production by strains of Pseudomonas aeruginosa using low cost raw materials. Biotechnol Prog 18:1277–1281
Ramos JL, Duque E, Gallegos MT, Godoy P, Ramos-Gonzalez MI, Rojas A, Teran W, Segura A (2002) Mechanisms of solvent tolerance in Gram-negative bacteria. Annu Rev Microbiol 56:743–768
Rodrigues L, Banat IM, Teixeira J, Oliveira R (2006) Biosurfactants: potential applications in medicine. J Antimicrob Chemother 57:609–618
Saitou N, Nei M (1987) The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol 4:406–425
Sandrin TR, Chech AM, Maier RM (2000) A rhamnolipid biosurfactant reduces cadmium toxicity during naphthalene biodegradation. Appl Environ Microbiol 66:4585–4588
Shin KH, Ahn Y, Kim KW (2005) Toxic effect of biosurfactant addition on the biodegradation of phenanthrene. Environ Toxicol Chem 24(11):2768–2774
Stacey SP, McLaughlin MJ, Cakmak I, Hettiarachchi GM, Scheckel KG, Karkkainen M (2008) Root uptake of lipophilic zinc–rhamnolipid complexes. J Agric Food Chem 56(6):2112–2117
Villaescusa I, Martinez M, Pilar M, Murat JC, Hostan C (1996) Toxicity of cadmium species in luminescent bacteria. Fresenius J Anal Chem 354:566–570
Wang X, Gong L, Liang S, Xiurong Han X, Zhu C, Li Y (2005) Algicidal activity of rhamnolipid biosurfactants produced by Pseudomonas aeruginosa. Harmful Algae 4:433–443
Zhang Y, Miller RM (1992) Enhanced octadecane dispersion and biodegradation by a Pseudomonas rhamnolipid surfactant (biosurfactant). Appl Environ Microbiol 58(10):3276–3282
Acknowledgments
This work was financially supported by the Estonian Ministry of Science and Education (targeted funding project SF0690063s08), Maj and Tor Nessling Foundation (grant no 2008416), Estonian Science Foundation (grant no 6974), and European Social Fund. We thank Prof. Henri-Charles Dubourguier for fruitful discussions, Thomas Leydier for help in chemical analysis of the samples, Sirje Vija for her advice in thin-layer chromatography, and Martin Romantshuk for P. fluorescens OS8 strain. PKSM Rahman thanks the Teesside University-sponsored Research and Enterprise Development Fund and Higher Education Innovation Fund (HEIF) for their support towards the completion of this project and the Bioscience for Business-Knowledge Transfer Network (BfB-KTN) for the award of “From Renewable Platform Chemicals to Value Added Products” (FROPTOP) fund to explore the biocatalytic study of biosurfactant production from renewable resources.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bondarenko, O., Rahman, P.K.S.M., Rahman, T.J. et al. Effects of Rhamnolipids from Pseudomonas aeruginosa DS10-129 on Luminescent Bacteria: Toxicity and Modulation of Cadmium Bioavailability. Microb Ecol 59, 588–600 (2010). https://doi.org/10.1007/s00248-009-9626-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00248-009-9626-5