Abstract
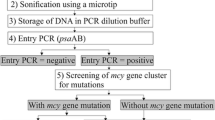
The filamentous cyanobacterium Planktothrix rubescens frequently occurs in deep and stratified lakes in the temperate region of the northern hemisphere and is a known producer of the hepatotoxic secondary metabolite microcystin. These cyclic heptapeptides are synthesized nonribosomally via large enzyme complexes encoded by the microcystin (mcy) synthetase gene cluster. The occurrence of cyanobacterial strains lacking microcystin, but containing the mcy gene cluster has been reported repeatedly; it was shown that this inactivation is due to mutations such as gene deletion events and the insertion of transposable elements. In the present study, 12 lakes in Austria, Germany, and Switzerland were sampled from July 2005 to October 2007, and the proportion of inactive mcy genotypes was quantified in relation to the total population of the red-pigmented filamentous cyanobacterium Planktothrix by means of quantitative polymerase chain reaction. In total, four different mutations were quantified, namely two insertions affecting mcyD, one insertion affecting mcyA, and a deletion within mcyH and mcyA. The mutations occurred over a wide range of population densities (40–570,000 filaments L−1), and their abundance was found to be positively correlated with population density. However, on average, all nontoxic mutants were found in a low proportion only (min 0%, mean 6.5% ± 1.1 (SE), max 52% of the total population). The genotype containing the mcyHA deletion had a significantly higher proportion (min 0%, mean 3.7% ± 1, max 52%) when compared with all the genotypes containing insertions within the mcy gene cluster (min 0%, mean 2.8% ± 0.7, max 24%). The results demonstrate that the occurrence of inactive mcy genotypes is linearly related to the population density, and selective sweeps of nontoxic mutants did not occur during the transition from prebloom to bloom conditions.





Similar content being viewed by others
References
Anagnostidis K, Komárek J (1988) Modern approach to the classification system of cyanophytes, 3-Oscillatoriales. Arch Hydrobiol Suppl Algol Stud 80(50–53):327–472
Barker GLA, Handley BA, Vacharapiyasophon P, Stevens JR, Hayes PK (2000) Allele-specific PCR shows that genetic exchange occurs among genetically diverse Nodularia (Cyanobacteria) filaments in the Baltic Sea. Microbiology 146:2865–2875
Berg OG, Kurland CG (2002) Evolution of microbial genomes: sequence acquisition and loss. Mol Biol Evol 19:2265–2276
Chorus I, Bartram J (1999) Toxic cyanobacteria in water. A guide to their public health consequences, monitoring and management. WHO, E & FN Spon, London, p 416
Christiansen G, Fastner J, Erhard M, Börner T, Dittmann E (2003) Microcystin biosynthesis in Planktothrix: genes, evolution, and manipulation. J Bacteriol 185:564–572
Christiansen G, Kurmayer R, Liu Q, Börner T (2006) Transposons inactivate biosynthesis of the nonribosomal peptide microcystin in naturally occurring Planktothrix spp. Appl Environ Microbiol 72:117–123
Christiansen G, Molitor C, Philmus B, Kurmayer R (2008) Nontoxic strains of cyanobacteria are the result of major gene deletion events induced by a transposable element. Mol Biol Evol 25:1695–1704
Edmondson WT, Litt AH (1982) Daphnia in Lake Washington. Limnol Oceanogr 27:272–293
Fastner J, Erhard M, von Döhren H (2001) Determination of oligopeptide diversity within a natural population of Microcystis (Cyanobacteria) by typing single colonies by matrix-assisted laser desorption ionization-time of flight mass spectrometry. Appl Environ Microbiol 67:5069–5076
Hesse K, Dittmann E, Börner T (2001) Consequences of impaired microcystin production for light-dependent growth and pigmentation of Microcystis aeruginosa PCC 7806. FEMS Microbiol Ecol 37:39–43
ISO (1992) Water quality - measurement of biochemical parameters - spectrometric determination of the chlorophyll-a concentration. International Organisation for Standardization, Geneve, p 12
Jacquet S, Briand J-F, Leboulanger C, Avois-Jacquet C, Oberhaus L, Tassin B, Vincon-Leite B, Paolini G, Druart J-C, Anneville O, Humbert J-F (2005) The proliferation of the toxic cyanobacterium Planktothrix rubescens following restoration of the largest natural French lake (Lac du Bourget). Harmful Algae 4:651–672
Kaebernick M, Rohrlack T, Christoffersen K, Neilan BA (2001) A spontaneous mutant of microcystin biosynthesis: genetic characterization and effect on Daphnia. Environ Microbiol 3:669–679
Kardinaal W, Tonk L, Janse I, Hol S, Slot P, Huisman J, Visser P (2007) Competition for light between toxic and nontoxic strains of the harmful cyanobacterium Microcystis. Appl Environ Microbiol 73:2939–2946
Kurmayer R, Christiansen G, Chorus I (2003) The abundance of microcystin-producing genotypes correlates positively with colony size in Microcystis and determines its microcystin net production in Lake Wannsee. Appl Environ Microbiol 69:787–795
Kurmayer R, Christiansen G, Fastner J, Börner T (2004) Abundance of active and inactive microcystin genotypes in populations of the toxic cyanobacterium Planktothrix spp. Environ Microbiol 6:831–841
Kurmayer R, Gumpenberger M (2006) Diversity of microcystin genotypes among populations of the filamentous cyanobacteria Planktothrix rubescens and Planktothrix agardhii. Mol Ecol 15:3849–3861
Kurmayer R, Jüttner F (1999) Strategies for the co-existence of zooplankton with the toxic cyanobacterium Planktothrix rubescens in Lake Zürich. J Plankt Res 21:659–683
Kurmayer R, Kutzenberger T (2003) Application of real-time PCR for quantification of microcystin genotypes in a population of the toxic cyanobacterium Microcystis sp. Appl Environ Microbiol 69:6723–6730
Ludwig W, Strunk O, Westram R, Richter L, Meier H, Yadhukumar, Buchner A, Lai T, Steppi S, Jobb G, Forster W, Brettske I, Gerber S, Ginhart AW, Gross O, Grumann S, Hermann S, Jost R, Konig A, Liss T, Lussmann R, May M, Nonhoff B, Reichel B, Strehlow R, Stamatakis A, Stuckmann N, Vilbig A, Lenke M, Ludwig T, Bode A, Schleifer KH (2004) ARB: a software environment for sequence data. Nucleic Acids Res 32:1363–1371
Mikalsen B, Boison G, Skulberg OM, Fastner J, Davies W, Gabrielsen TM, Rudi K, Jakobsen KS (2003) Natural variation in the microcystin synthetase operon mcyABC and impact on microcystin production in Microcystis strains. J Bacteriol 185:2774–2785
Mira A, Ochman H, Moran NA (2001) Deletional bias and the evolution of bacterial genomes. Trends Genet 17:589–596
Naselli-Flores L, Barone R, Chorus I, Kurmayer R (2007) Toxic cyanobacterial blooms under a semiarid mediterranean climate: The magnification of a problem. Environ Toxicol 22:399–404
Nishizawa T, Asayama M, Fujii K, Harada K, Shirai M (1999) Genetic analysis of the peptide synthetase genes for a cyclic heptapeptide microcystin in Microcystis spp. J Biochem 126:520–529
Nürnberg GK, LaZerte BD (2003) An artificially induced Planktothrix rubescens surface bloom in a small kettle lake in Southern Ontario compared to blooms world-wide. Lake Res Manag 19:307–322
Padisák J, Scheffler W, Kasprzak P, Koschel R, Krienitz L (2003) Interannual variability in the phytoplankton composition of Lake Stechlin (1994-2000). Arch Hydrobiol/Advanc Limnol 58:101–133
Pridmore R, Etheredge M (1987) Planktonic cyanobacteria in New Zealand inland waters: distribution and population dynamics. N Z J Mar Freshw Res 21:491–502
Rantala A, Fewer DP, Hisbergues M, Rouhiainen L, Vaitomaa J, Börner T, Sivonen K (2004) Phylogenetic evidence for the early evolution of microcystin synthesis. Proc Natl Acad Sci USA 101:568–573
Rippka R (1988) Isolation and purification of cyanobacteria. Meth Enzymol 167:3–27
Rohrlack T, Dittmann E, Henning M, Börner T, Kohl J-G (1999) Role of microcystins in poisoning and food ingestion inhibition of Daphnia galeata caused by the cyanobacterium Microcystis aeruginosa. Appl Environ Microbiol 65:737–739
Rohrlack T, Edvardsen B, Skulberg R, Halstvedt CB, Utkilen HC, Ptacnik R, Skulberg OM (2008) Oligopeptide chemotypes of the toxic freshwater cyanobacterium Planktothrix can form subpopulations with dissimilar ecological traits. Limnol Oceanogr 53:1279–1293
Rouhiainen L, Vakkilainen T, Siemer BL, Buikema W, Haselkorn R, Sivonen K (2004) Genes coding for hepatotoxic heptapeptides (microcystins) in the cyanobacterium Anabaena strain 90. Appl Environ Microbiol 70:686–692
Schober E, Kurmayer R (2006) Evaluation of different DNA sampling techniques for the application of the real-time PCR method for the quantification of cyanobacteria in water. Lett Appl Microbiol 42:412–417
Schober E, Werndl M, Laakso K, Korschinek I, Sivonen K, Kurmayer R (2007) Interlaboratory comparison of Taq Nuclease Assays for the quantification of the toxic cyanobacteria Microcystis sp. J Microbiol Meth 69:122–128
Siguier P, Perochon J, Lestrade L, Mahillon J, Chandler M (2006) ISfinder: the reference centre for bacterial insertion sequences. Nucl Acid Res 34:D32–D36
Sokal R, Rohlf F (1995) Biometry. The principles and practice of statistics in biological research, 3rd edn. W.H. Freeman and Company, New York, p 886
Suda S, Watanabe MM, Otsuka S, Mahakahant A, Yongmanitchai W, Nopartnaraporn N, Liu Y, Day JG (2002) Taxonomic revision of water-bloom-forming species of oscillatorioid cyanobacteria. Int J Syst Evol Microbiol 52:1577–1595
Tillett D, Dittmann E, Erhard M, vonDöhren H, Börner T, Neilan BA (2000) Structural organization of microcystin biosynthesis in Microcystis aeruginosa PCC7806: an integrated peptide-polyketide synthetase system. Chem Biol 7:753–764
Tillett D, Parker DL, Neilan BA (2001) Detection of toxigenicity by a probe for the microcystin synthetase A gene (mcyA) of the cyanobacterial genus Microcystis: comparison of toxicities with 16S rRNA and phycocyanin operon (phycocyanin intergenic spacer) phylogenies. Appl Environ Microbiol 67:2810–2818
Travisano M, Velicer GJ (2004) Strategies of microbial cheater control. Trends Microbiol 12:72–78
Utermöhl H (1958) Zur Vervollkommnung der quantitativen Phytoplanktonmethodik. Mitt Internat Verein Limnol 2:1–38
Vollenweider RA, Kerekes J (1982) Eutrophication of waters. Monitoring, assessment and control. OECD Cooperative programme on monitoring of inland waters (Eutrophication control). Environmental Directorate, OECD, Paris, p 154
Walsby AE, Avery A (1996) Measurement of filamentous cyanobacteria by image analysis. J Microbiol Meth 26:11–20
Walsby AE, Ng G, Dunn C, Davis PA (2004) Comparison of the depth where Planktothrix rubescens stratfies and the depth where the daily insolation supports its neutral buoyancy. New Phytol 162:133–145
Welker M, Christiansen G, von Döhren H (2004) Diversity of coexisting Planktothrix (cyanobacteria) chemotypes deduced by mass spectral analysis of microystins and other oligopeptides. Arch Microbiol 182:288–298
Welker M, Erhard M (2007) Consistency between chemotyping of single filaments of Planktothrix rubescens (Cyanobacteria) by MALDI-TOF and the peptide patterns of strains determined by HPLC-MS. J Mass Spectrom 42:1062–1068
Wetzel RG, Likens GE (2000) Limnological analyses, 3rd edn. Springer-Verlag, New York
Acknowledgements
We would like to thank Guntram Christiansen for his help in molecular biological techniques. We are most grateful to Arno Stöckli (Department Bau, Verkehr und Umwelt, Kanton Aarau, Switzerland), Ferdinand Schanz (University of Zürich), Günther Bruschek and Karl Mayrhofer (BAW Scharfling, Austria) for the provision of water samples. Marcel Erhard did the automated MALDI-TOF MS measurements on peptide composition in single filaments. We appreciated the comments of two anonymous reviewers. This study was financed by the Austrian Science Fund project P18185 “Microevolution of toxin synthesis in cyanobacteria”.
Author information
Authors and Affiliations
Corresponding author
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Fig. S1
Relationship between Planktothrix biovolume as determined by the TNA and estimated from the Lugol fixed samples using the sedimentation method. For details on the regression curve, see text (DOC 242 kb)
Fig. S2
Relationship between the Planktothrix biovolume as determined by 16S rDNA-TNA and the biovolume of genotypes containing the IS element as determined by TNA (TIB). For details on the regression curve, see text (DOC 379 kb)
Rights and permissions
About this article
Cite this article
Ostermaier, V., Kurmayer, R. Distribution and Abundance of Nontoxic Mutants of Cyanobacteria in Lakes of the Alps. Microb Ecol 58, 323–333 (2009). https://doi.org/10.1007/s00248-009-9484-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00248-009-9484-1