Abstract
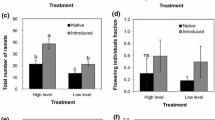
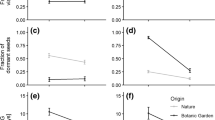
Cool-season grasses often harbor obligate fungal symbionts from the genus Neotyphodium, and these symbiota can function as a single ecological unit. Previous studies have shown that gene flow in Neotyphodium in Festuca arizonica is low enough such that populations could diverge and form local adaptations. A reciprocal transplant experiment was performed between two F. arizonica/Neotyphodium populations in Arizona, Clint’s Well and Flagstaff, using symbiota with the most common Neotyphodium genotypes in each population, to test for local adaptations. The genetic difference between populations is potentially large as Neotyphodium from Clint’s Well are of hybrid origin. Local environmental variation was the most important source of variation for F. arizonica/Neotyphodium symbiota growth, with individuals at Flagstaff growing larger and individuals at Clint’s Well not reproducing. Local environment and the source population of the symbiota interacted to affect vegetative growth. Symbiota from Clint’s Well, which harbor hybrid Neotyphodium, had higher volume/wet mass and volume/dry mass ratios but only in the marginal Clint’s Well habitat. The local environment also affected F. arizonica/Neotyphodium reproduction because only symbiota transplanted to Flagstaff reproduced. Symbiota from Clint’s Well produced more panicles, whereas symbiota from Flagstaff with nonhybrid Neotyphodium produced greater seed mass per panicle. Overall seed mass production was not different, suggesting that the two strategies are functionally equivalent. We find that F. arizonica/Neotyphodium symbiota vary geographically, but potential local adaptations are only apparent in marginal habitats and may be related to the evolutionary history of the Neotyphodium part of the symbiota.



Similar content being viewed by others
References
Thompson JN (1994) The coevolutionary process. University of Chicago Press, Chicago
Gandon S (1998) Local adaptation and host–parasite interactions. Trends Ecol Evol 13:214–216
Gandon S (2002) Local adaptation and the geometry of host–parasite coevolution. Ecol Lett 5:246–256
Gandon S, Capowiez Y, Dubois Y, Michalakis Y, Olivieri I (1996) Local adaptation and gene-for-gene coevolution in a metapopulation model. Proc R Soc Lond B Biol Sci 263:1003–1009
Nuismer SL, Thompson JN, Gomulkiewicz R (1999) Gene flow and geographically structured coevolution. Proc R Soc Lond B Biol Sci 266:605–609
Nuismer SL, Thompson JN, Gomulkiewicz R (2000) Coevolutionary clines across selection mosaics. Evolution 54:1102–1115
Parker MA (1995) Plant fitness variation caused by different mutualist genotypes. Ecology 76:1525–1535
Wilkinson HH, Parker MA (1996) Symbiotic specialization and the potential for genotypic coexistence in a plant–bacterial mutualism. Oecologia 108:361–367
Freeman S, Rodriguez RJ (1993) Genetic conversion of a fungal plant pathogen to a nonpathogenic, endophytic mutualist. Science 260:75–78
Douglas AE, Smith DC (1983) The cost of symbionts to their host in green hydra. In: Schenk HEA, Schwemmler W (eds) Endocytobiology II. Walter de Gruyter, Berlin, pp 631–648
Johnson NC, Graham JH, Smith FA (1997) Functioning of mycorrhizal associations along the mutualism–parasitism continuum. New Phytol 135:575–586
Cushman JH, Whitham TG (1989) Conditional mutualism in a membracid ant association - temporal, age-specific, and density-dependent effects. Ecology 70:1040–1047
Pellmyr O, Huth CJ (1994) Evolutionary stability of mutualism between yuccas and yucca moths. Nature 372:257–260
Burdon JJ, Thrall PH (1999) Spatial and temporal patterns in coevolving plant and pathogen associations. Am Nat 153:S15–S33
Bush LP, Wilkinson HH, Schardl CL (1997) Bioprotective alkaloids of grass–fungal endophyte symbioses. Plant Physiol 114:1–7
Faeth SH (2002) Are endophytic fungi defensive plant mutualists? Oikos 98:25–36
Elbersen HW, West CP (1996) Growth and water relations of field-grown tall fescue as influenced by drought and endophyte. Grass Forage Sci 51:333–342
Elmi AA, West CP (1995) Endophyte infection effects on stomatal conductance, osmotic adjustment and drought recovery of tall fescue. New Phytol 131:61–67
Hill NS, Pachon JG, Bacon CW (1996) Acremonium coenophialum mediated short- and long-term drought acclimation in tall fescue. Crop Sci 36:665–672
Malinowski D, Leuchtmann A, Schmidt D, Nosberger J (1997) Growth and water status in meadow fescue is affected by Neotyphodium and Phialophora species endophytes. Agron J 89:673–678
Vila-Aiub MM, Martinez-Ghersa MA, Ghersa CM (2003) Evolution of herbicide resistance in weeds: vertically transmitted fungal endophytes as genetic entities. Evol Ecol 17:441–456
Zabalgogeazcoa I, Ciudad AG, de Aldana BR, Criado BG (2006) Effects of the infection by the fungal endophyte Epichloe festucae in the growth and nutrient content of Festuca rubra. Eur J Agron 24:374–384
Malinowski DP, Belesky DP (2000) Adaptations of endophyte-infected cool-season grasses to environmental stresses: mechanisms of drought and mineral stress tolerance. Crop Sci 40:923–940
Arachevaleta M, Bacon CW, Hoveland CS, Radcliffe DE (1989) Effect of the Tall fescue endophyte on plant response to environmental stress. Agron J 81:83–90
Saikkonen K, Lehtonen P, Helander M, Koricheva J, Faeth SH (2006) Model systems in ecology: dissecting the endophyte–grass literature. Trends Plant Sci 11:428–433
Cheplick GP, Clay K, Marks S (1989) Interactions between infection by endophytic fungi and nutrient limitation in the grasses Lolium perenne and Festuca arundinacea. New Phytol 111:89–97
Saikkonen K, Faeth SH, Helander M, Sullivan TJ (1998) Fungal endophytes: a continuum of interactions with host plants. Annu. Rev Ecol Syst 29:319–343
Bultman TL, Bell G, Martin WD (2004) A fungal endophyte mediates reversal of wound-induced resistance and constrains tolerance in a grass. Ecology 85:679–685
Lehtonen P, Helander M, Wink M, Sporer F, Saikkonen K (2005) Transfer of endophyte-origin defensive alkaloids from a grass to a hemiparasitic plant. Ecol Lett 8:1256–1263
Cheplick GP (1998) Genotypic variation in the regrowth of Lolium perenne following clipping: effects of nutrients and endophytic fungi. Funct Ecol 12:176–184
Faeth SH, Bush LP, Sullivan TJ (2002) Peramine alkaloid variation in Neotyphodium-infected Arizona fescue: effects of endophyte and host genotype and environment. J Chem Ecol 28:1511–1526
Faeth SH, Sullivan TJ (2003) Mutualistic, asexual endophytes in a native grass are usually parasitic. Am Nat 161:310–325
Brem D, Leuchtmann A (2001) Epichloe grass endophytes increase herbivore resistance in the woodland grass Brachypodium sylvaticum. Oecologia 126:522–530
Tintjer T, Rudgers JA (2006) Grass–herbivore interactions altered by strains of a native endophyte. New Phytol 170:513–521
Faeth SH, Gardner DR, Hayes CJ, Jani A, Wittlinger SK, Jones TA (2006) Temporal and spatial variation in alkaloid levels in Achnatherum robustum, a native grass infected with the endophyte Neotyphodium. J Chem Ecol 32:307–324
Brem D, Leuchtmann A (2003) Molecular evidence for host-adapted races of the fungal endophyte Epichloe bromicola after presumed host shifts. Evolution 57:37–51
Hesse U, Schoberlein W, Wittenmayer L, Forster K, Warnstorff K, Diepenbrock W, Merbach W (2003) Effects of Neotyphodium endophytes on growth, reproduction and drought-stress tolerance of three Lolium perenne L. genotypes. Grass Forage Sci 58:407–415
Brem D, Leuchtmann A (2002) Intraspecific competition of endophyte infected vs uninfected plants of two woodland grass species. Oikos 96:281–290
Saikkonen K, Ion D, Gyllenberg M (2002) The persistence of vertically transmitted fungi in grass metapopulations. Proc R Soc Lond B Biol Sci 269:1397–1403
Tsai HF, Liu JS, Staben C, Christensen MJ, Latch GCM, Siegel MR, Schardl CL (1994) Evolutionary diversification of fungal endophytes of tall fescue grass by hybridization with Epichloë species. Proc Natl Acad Sci USA 91:2542–2546
Schardl CL, Leuchtmann A, Tsai HF, Collett MA, Watt DM, Scott DB (1994) Origin of a fungal symbiont of perennial ryegrass by interspecific hybridization of a mutualist with the ryegrass choke pathogen, Epichloë typhina. Genetics 136:1307–1317
Moon CD, Craven KD, Leuchtmann A, Clement SL, Schardl CL (2004) Prevalence of interspecific hybrids amongst asexual fungal endophytes of grasses. Mol Ecol 13:1455–1467
Christensen MJ, Leuchtmann A, Rowan DD, Tapper BA (1993) Taxonomy of Acremonium endophytes of tall fescue (Festuca arundinacea), meadow fescue (F. pratensis) and perennial ryegrass (Lolium perenne). Mycol Res 97:1083–1092
Moon CD, Scott B, Schardl CL, Christensen MJ (2000) The evolutionary origins of Epichloë endophytes from annual ryegrasses. Mycologia 92:1103–1118
Craven KD, Blankenship JD, Leuchtmann A, Hignight K, Schardl CL (2001) Hybrid fungal endophytes symbiotic with the grass Lolium pratense. Sydowia 53:44–73
Moon CD, Miles CO, Jarlfors U, Schardl CL (2002) The evolutionary origins of three new Neotyphodium endophyte species from grasses indigenous to the Southern Hemisphere. Mycologia 94:694–711
Sullivan TJ, Faeth SH (2004) Gene flow in the endophyte Neotyphodium and implications for coevolution with Festuca arizonica. Mol Ecol 13:649–656
Selosse MA, Schardl CL (2007) Fungal endophytes of grasses: hybrids rescued by vertical transmission? An evolutionary perspective. New Phytol 173:452–458
Clay K, Schardl CL (2002) Evolutionary origins and ecological consequences of endophyte symbiosis with grasses. Am Nat 160:S99–S127
Schardl CL, Craven KD (2003) Interspecific hybridization in plant-associated fungi and oomycetes: a review. Mol Ecol 12:2861–2873
Kearney TH, Peebles RH (1960) Arizona Flora. University of California Press, Berkeley, CA
Schulthess FM, Faeth SH (1998) Distribution, abundances, and associations of the endophytic fungal community of Arizona fescue (Festuca arizonica). Mycologia 90:569–578
Saikkonen K, Helander M, Faeth SH, Schulthess F, Wilson D (1999) Endophyte–grass–herbivore interactions: the case of Neotyphodium endophytes in Arizona fescue populations. Oecologia 121:411–420
Lopez JE, Faeth SH, Miller M (1995) Effect of endophytic fungi on herbivory by redlegged grasshoppers (Orthoptera: Acrididae) on Arizona fescue. Environ Entomol 24:1576–1580
Tibbets TM, Faeth SH (1999) Neotyphodium endophytes in grasses: deterrents or promoters of herbivory by leaf-cutting ants? Oecologia 118:297–305
An ZQ, Liu JS, Siegel MR, Bunge G, Schardl CL (1992) Diversity and origins of endophytic fungal symbionts of the North American grass Festuca arizonica. Theor Appl Genet 85:366–371
Cabral D, Cafaro MJ, Saidman B, Lugo M, Reddy PV, White JF (1999) Evidence supporting the occurrence of a new species of endophyte in some South American grasses. Mycologia 91:315–325
White JF, Cole GT, Morganjones G (1987) Endophyte-host associations in forage grasses. VI. A new species of Acremonium isolated from Festuca arizonica. Mycologia 79:148–152
US Department of Agriculture (1967) General soil map—Coconino county, Arizona. US Department of Agriculture—Soil Conservation Service, Washington, DC
Moore KJ, Moser LE (1995) Quantifying developmental morphology of perennial grasses. Crop Sci 35:37–43
Siegel MR, Bush LP (1996) Defensive chemicals in grass-fungal endophyte associations. In: Romero JT, Saunders JA, Barbosa P (eds) Phytochemical diversity and redundancy in ecological interactions, vol. 30. Plenum, New York, pp 81–117
SAS Institute (2003) JMP IN. SAS Institute, Cary, NC
Rieseberg LH (1997) Hybrid origins of plant species. Annu Rev Ecol Syst 28:359–389
Singer JW (2002) Species and nitrogen effect on growth rate, tiller density, and botanic composition in grass hay production. Crop Sci 42:208–214
Sullivan TJ (2003) Geographic and genetic variation in the Neotyphodium/Festuca arizonica interaction. Ph.D. dissertation
Acknowledgments
The authors wish to thank Natalie Fuller and Phil Steiner for their field assistance and Dr. Thomas Dowling for his aid with the microsatellites and DNA sequencing. We also thank Andrea Jani and Tamaru Hunt-Joshi for their helpful comments on the manuscript. This research was supported by grants from the Associated Graduate Students of A.S.U. and by an anonymous donor to the ASU foundation to TJS and DEB 0128343 to SHF.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sullivan, T.J., Faeth, S.H. Local Adaptation in Festuca arizonica Infected by Hybrid and Nonhybrid Neotyphodium Endophytes. Microb Ecol 55, 697–704 (2008). https://doi.org/10.1007/s00248-007-9312-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00248-007-9312-4