Abstract
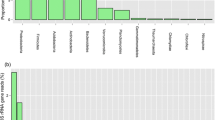
To examine bacterial community composition in rhizosphere of plants colonizing on mine tailings and phylogenetic differences between subcommunities resistant to different metals, we constructed four clone libraries of 16S rDNA sequences. One was amplified directly from tailing microbial DNA (named as Ci library) and three from cultures on the plates containing of 0.5 mM CdCl2 (Cd library), 2 mM Pb (NO3)2 (Pb library), and without any metals (Cw library). In total, nine bacterial divisions and two unclassified groups were identified from 352 clones of these libraries. Ci clones covered eight divisions, whereas all cultivable clones only covered four divisions. Thus, Ci library provided more phylogenetic diversity than cultivable libraries. However, the microbes represented by the cultivable clones were more similar to previously described bacteria than those represented by Ci clones. All Ci clones were not found in three cultivable libraries. Cd library were exclusively Gram-negative bacteria of Acinetobacter, Ralstonia, Comamonas, and Chryseobacterium. Meanwhile, dominant Gram-positive bacteria in Pb library, Paenibacillus and Bacillus, were also not found in Cd library. Our data indicate that phylogenetic structure was very different from those in acid mine drainage. Meanwhile, tailings harbored phylogenetically distinct subcommunities resistant to Pb and Cd.


Similar content being viewed by others
References
Anderson, RT, Vrionis, HA, Ortiz-Bernad, I, Resch, CT, Long, PE, Dayvault, R, Krp, K, Marutzky, S, Metzler, DR, Peacock, A, White, DC, Lowe, M, Lovley, DR (2003) Stimulating the in situ activity of Geobacter species to remove uranium from the groundwater of a uranium-contaminated aquifer. Appl Environ Microbiol 69: 5884–5891
Axelrood, PE, Chow, ML, Radomski, CC, McDermott, JM, Davies, J (2002) Molecular characterization of bacterial diversity from British Columbia forest soils subjected to disturbance. Can J Microbiol 48: 655–674
Brown, GE, Foster, AL, Ostergren, JD (1999) Mineral surfaces and bioavailability of heavy metals: a molecular-scale perspective. Proc Natl Acad Sci U S A 96: 3388–3395
Canovas, D, Cases, I, de Lorenzo, V (2003) Heavy metal tolerance and metal homeostasis in Pseudomonas putida as revealed by complete genome analysis. Environ Microbiol 5: 1242–1256
Chang, YJ, Peacock, AD, Long, PE, Stephen, JR, McKinley, JP, Macnaughton, SJ, Hussain, AKMA, Saxton, AM, White, DC (2001) Diversity and characterization of sulfate-reducing bacteria in groundwater at a uranium mill tailings site. Appl Environ Microbiol 67: 3149–3160
Dhakephalkar, PK, Chopade, BA (1994) High levels of multiple metal resistance and its correlation to antibiotic resistance in environmental isolates of Acinetobacter. Biometals 7: 67–74
Doleman, P, Jansen, E, Michels, M, van Til, M (1994) Effects of heavy metals in soil on microbial diversity and activity as shown by the sensitivity-resistance index, an ecologically relevant parameter. Biol Fertil Soils 17: 177–184
Dudka, S, Adriano, DC (1997) Environmental impacts of metal ore mining and processing: a review. J Environ Qual 26: 590–602
Dunbar, J, Takala, S, Barns, SM, Davis, JA, Kuske, CR (1999) Levels of bacterial community diversity in four arid soils compared by cultivation and 16S rRNA gene cloning. Appl Environ Microbiol 65: 1662–1669
Ellis, RJ, Morgan, P, Weightman, AJ, Fry, JC (2003) Cultivation-dependent and -independent approaches for determining bacterial diversity in heavy-metal-contaminated soil. Appl Environ Microbiol 69: 3223–3230
Filion, M, Hamelin, RC, Bernier, L, St-Arnaud, M (2004) Molecular profiling of rhizosphere microbial communities associated with healthy and diseased black spruce (Picea mariana) seedlings grown in a nursery. Appl Environ Microbiol 70: 3541–3551
Fortin, D, Davis, B, Southham, G, Beveridge, TJ (1995) Biogeochemical phenomena induced by bacteria within sulfidic mine tailings. J Ind Microbiol 14: 178–185
Gadd, GM (2000) Bioremedial potential of microbial mechanisms of metal mobilization and immobilization. Curr Opin Biotechnol 11: 271–279
Good, IJ (1953) The population frequencies of species and the estimation of population parameters. Biometrika 40: 237–264
Holden, PA, Firestone, MK (1997) Soil microorganisms in soil cleanup: how can we improve our understanding? J Environ Qual 26: 32–40
Hugenholtz, P, Goebel, BM, Pace, NR (1998) Impact of culture–independent studies on the emerging phylogenetic view of bacterial diversity. J Bacteriol 180: 4765–4774
Jackson, CR, Harrison, KG, Dugas, SL (2005) Enumeration and characterization of culturable arsenate resistant bacteria in a large estuary. Syst Appl Microbiol 28: 727–734
Kelly, JJ, Häggblom, MM, Tate, RL (2003) Effects of heavy metal contamination and remediation on soil microbial communities in the vicinity of a zinc smelter as indicated by analysis of microbial community phospholipids fatty acid profiles. Biol Fertil Soils 38: 65–71
Lasat, MM (2002) Phytoextraction of toxic metals: a review of biological mechanisms. J Environ Qual 31: 109–120
Mahmoud, KK, Leduc, LG, Ferroni, GD (2005) Detection of Acidithiobacillus ferrooxidans in acid mine drainage environments using fluorescent in situ hybridization (FISH). J Microbiol Methods 61: 33–45
Nemergut, DR, Martin, AP, Schmidt, SK (2004) Intergon diversity in heavy-metal-contaminated mine tailings and inferences about integron evolution. Appl Environ Microbiol 70: 1160–1168
Nies, DH (1995) The cobalt, zinc, and cadmium efflux system CzcABC from Alcaligenes eutrophus functions as a cation–proton antiporter in Escherichia coli. J Bacteriol 177: 2707–2712
Nübel, U, Engelen, B, Felske, A, Snaidr, J, Wieshuber, A, Amann, RI, Ludwig, W, Backhaus, H (1996) Sequence heterogeneities of genes encoding 16S rRNAs in Paenibacillus polymyxa detected by temperature gradient gel electrophoresis. J Bacteriol 178: 5636–5643
Oger, C, Mahillon, J, Petit, F (2003) Distribution and diversity of a cadmium resistance (cadA) determinant and occurrence of IS257 insertion sequences in Staphylococcal bacteria isolated from a contaminated estuary (Seine, France). FEMS Microbiol Ecol 43: 173–183
Pennanen, T, Frostegåd, Å, Fritze, H, Bååth, E (1998) Phospholipid fatty acid composition and heavy metal tolerance of soil microbial communities along two heavy metal-polluted gradients in coniferous forests. Appl Environ Microbiol 62: 420–428
Ranjard, L, Brother, L, Nazaret, S (2000) Sequencing bands of ribosomal intergenic spacer analysis fingerprints for characterization and microscale distribution of soil bacterium populations responding to mercury spiking. Appl Environ Microbiol 66: 5334–5339
Schippers, A, Hallmann, R, Wentzien, S, Sand, W (1995) Microbial diversity in uranium mine waste heaps. Appl Environ Microbiol 61: 2930–2935
Schloss, PD, Handelsman, J (2005) Introducing DOTUR, a computer program for defining operational taxonomic units and estimating species richness. Appl Environ Microbiol 71: 1501–1506
Schloss, PD, Larget, BR, Handelsman, J (2004) Integration of microbial ecology and statistics: a test to compare gene libraries. Appl Environ Microbiol 70: 5485–5492
Shayne, J, Hugenholtz, P, Sangwan, P, Osborne, CA, Janssen, PH (2003) Laboratory cultivation of widespread and previously uncultured soil bacteria. Appl Environ Microbiol 69: 7210–7215
Southham, G, Beveridge, TJ (1992) Enumeration of thiobacilli within pH-neutral and acidic mine tailings and their role in the development of secondary mineral soil. Appl Environ Microbiol 58: 1904–1912
Timoney, JF, Port, J, Giles, J, Spanier, J (1978) Heavy–metal and antibiotic resistance in the bacterial flora of sediments of New York Bight. Appl Environ Microbiol 36: 465–472
von der Weid, I, Paiva, E, Nobrega, A, van Elsas, JD, Seldin, L (2000) Diversity of Paenibacillus polymyxa strains isolated from the rhizosphere of maize planted in Cerrado soil. Res Microbiol 151: 369–381
Wilcke W, Kretzschmar S, Bundt M, Zech W (1999) Metal concentrations in aggregate interiors, exteriors, whole aggregates, and bulk of Costa Rican soils. Soil Sci Soc Am J 63: 1244–1249
Zhang, H, Sekiguchi, Y, Hanada, S, Hugenholtz, P, Kim, H, Kamagata, Y, Nakamura, K (2003) Gemmatimonas aurantiaca gen. nov., sp. nov., a Gram-negative, aerobic, polyphosphate accumulating microorganism, the first cultured representative of the new bacterial phylum Gemmatimonadetes phyl. nov. Int J Syst Evol Microbiol 53: 1155–1163
Zhang, H, Duan, C, Shao, Q, Ren, W, Sha, T, Cheng, L, Zhao, Z, Hu, B (2004) Genetic and physiological diversity of phylogenetically and geographically distinct groups of Arthrobacter isolated from lead–zinc mine tailings. FEMS Microbiol Ecol 49: 333–341
Acknowledgements
We thank Zhi-Ying Zhu, Chan-Wen Xu, Yan Li, Shu-Hua Ge, Tao Li, and Chang-Chong Liang in Yunnan University for sampling, library constructions, and RFLP analyses. This study was funded by the Natural Science Foundation of China (30560033) and the National Program on Key Basic Research Projects of China (special item 2005CCA05700).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhang, HB., Yang, MX., Shi, W. et al. Bacterial Diversity in Mine Tailings Compared by Cultivation and Cultivation-independent Methods and their Resistance to Lead and Cadmium. Microb Ecol 54, 705–712 (2007). https://doi.org/10.1007/s00248-007-9229-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00248-007-9229-y