Abstract
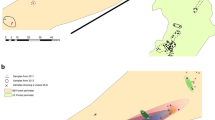
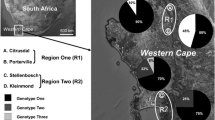
Epichloë species are systemic fungal endophytes that usually specialize in a certain group of related grass species. We examined the infection frequency of Epichloë festucae in populations of two fine fescue species (Festuca rubra and F. ovina) in natural and seminatural habitats at 86 study sites (total = 2514 plants) across Finland and northern Norway. Infection incidence varied significantly among grass species and populations. A substantial number of the F. rubra and F. ovina populations (53 out of 77 and 25 out of 30, respectively) were either endophyte-free or had very low (<20%) infection frequencies. The highest infection frequencies were found in subarctic areas. Moreover, infection incidence differed between habitats. In the area with the highest infection frequencies, we used microsatellite markers to study genetic diversity and the rates of gene flow of E. festucae among 12 F. rubra populations. Twenty out of the 25 fungal genotypes detected with four microsatellite markers were carrying multiple alleles in at least one locus, indicating multiple infections or vegetative hybridization of the fungus. One dominant genotype occurred in all 12 populations, representing 63.5% of all isolates. We found a moderate level of average genotypic variation and a low level of genetic differentiation (F st = 0.0814). There was no correlation between infection frequency and genotypic diversity. Although the existence of a dominant genotype and the detected linkage disequilibrium suggest that the fungus is mainly asexual and vertically transmitted, the multiallelic loci and variation of genetic diversity among populations indicate occasional contagious spread and sexual or parasexual recombination of the fungus in some populations. Furthermore, the genotypes carrying multiallelic loci suggest the possibility of multiple infections or hybridization of the endophyte.



Similar content being viewed by others
References
Agapow, PM, Burt, A (2000) Multi-locus 1.2. Dept of Biology, Imperial College, Silwood Park, Ascot, Berks, SL57PY, UK
Ahlholm, JU, Helander, M, Lehtimäki, S, Wäli, P, Saikkonen, K (2002b) Vertically transmitted fungal endophytes: different responses of host–parasite systems to environmental conditions. Oikos 99: 173–183
Arroyo García, R, Martínez Zapater, JM, García Criado, B, Zabalgogeazcoa, I (2002) Genetic structure of natural populations of the grass endophyte Epichloë festucae in semiarid grasslands. Mol Ecol 11: 355–364
Bacon, CW, Hinton, DM (1991) Microcyclic conidiation cycles in Epichloë typhina. Mycologia 83: 743–751
Bazely, DR, Vicari, M, Emmerich, S, Filip, L, Lin, D, Inman, A (1997) Interactions between herbivores and endophyte-infected Festuca rubra from Scottish islands of St. Kilda, Benbecula and Rum. J Appl Ecol 34: 847–860
Bierzychudeck, P (1985) Patterns in plant parthenogenesis. Experientia 41: 1255–1264
Brem, D, Leuchtmann, A (2003) Molecular evidence for host-adapted races of the fungal endophyte Epichloë bromicola after presumed host shifts. Evolution 57: 37–51
Brown, AHD, Feldman, MW Nevo E (1980) Multi-locus structure of natural populations of Hordeum spontaneum. Genetics 96: 523–536
Bucheli, E, Leuchtmann, A (1996) Evidence for genetic differentiation between choke-inducing and asymptomatic strains of the Epichloe grass endophyte from Brachypodium sylvaticum. Evolution 50: 1879–1887
Burt, A, Carter, DA, Koenig, GL, White, TJ, Taylor, JW (1996) Molecular markers reveal cryptic sex in the human pathogen Coccidioides immitis. Proc Natl Acad Sci USA 93: 770–773
Cheplick, GP (1989) Interactions between infection by endophytic fungi and nutrient limitation in the grasses Lolium perenne and Festuca arundinacea. New Phytol 111: 89–97
Christensen, MJ, Simpson, WR, Al Samarrai, T (2000) Infection of tall fescue and perennial ryegrass plants by combinations of different Neotyphodium endophytes. Mycol Res 104: 974–978
Clay, K (1987) Effects of fungal endophytes on the seed and seedling biology of Lolium perenne and Festuca arundinacea. Oecologia 73: 358–362
Clay, K (1993) The ecology and evolution of endophytes. Agric Ecosyst Environ 44: 39–64
Clay, K, Holah, J (1999) Fungal endophyte symbiosis and plant diversity in successional fields. Science 285: 1742–1744
Clay, K, Schardl, C (2002) Evolutionary origins and ecological consequences of endophyte symbiosis with grasses. Am Nat 160: S99–S127
Dybdahl, MF, Lively, CM (1996) The geography of coevolution: comparative population structures for a snail and its trematode parasite. Evolution 50: 2264–2275
Elamo, P (2002) Birch rust and endophytic fungi in birch leaves: effects of host plant genetic background and environmental factors. Annales Universitatis Turkuensis. ser. aII. tom. 131. Univ. of Turku, Turku, Finland
Geiser, DM, Pitt, JI, Taylor JW (1998) Cryptic speciation and recombination in the aflatoxin-producing fungus Aspergillus flavus. Proc Natl Acad Sci USA 95: 388–393
Goudet, J (2002) Fstat ver. 2.9.3.2. Institute of Ecology, Biology Building, UNIL, CH-1015, Lausanne, Switzerland
Gräser, Y, Volovsek, M, Arrington, J, Schönian, G, Presber, W, Mitchell, TG, Vilgalys, R (1996) Molecular markers reveal that population structure of the human pathogen Candida albicans exhibits both clonality and recombination. Proc Natl Acad Sci USA 93: 12473–12477
Halkett, F, Simon JC, Balloux F (2005) Tackling the population genetics of clonal and partially clonal organisms. Trends Ecol Evol 20: 194–201
Harberd, DJ (1961) Observations on population structure and longevity of Festuca rubra L. New Phytol 60: 184–192
Hedrick, PW (2005) Genetics of Populations, 3rd edn. Jones and Bartlett Publishers, Sudbury, MA, USA
Keiper, FJ, Hayden, MJ, Park, RF, Wellings, CR (2003) Molecular genetic variability of Australian isolates of five cereal rust pathogens. Mycol Res 107: 545–556
Kirby, EJM (1961) Host–parasite relations in the choke disease of grasses. Trans Br Mycol Soc 44: 493–503
Kohli, Y, Kohn, LM (1998) Random association among alleles in clonal populations of Sclerotinia sclerotiorum. Fungal Genet Biol 23: 139–149
Koponen, H, Mäkelä, K (1976) Phyllachora graminis, P. silvatica, Epichloë typhina and Acrospermum graminum on grasses in Finland. Karstenia 15: 46–55
Lehtonen, P, Helander, M, Saikkonen, K (2005) Are endophyte-mediated effects on herbivores conditional on soil nutrients? Oecologia 142: 38–45
Leuchtmann, A, Clay, K (1997) The population biology of grass endophytes. In: Carroll, GC, Tudzynski, P (Eds.) The Mycota. V. Plant relationships, Part B. Springer-Verlag, Berlin, pp 185–204
Leuchtmann, A, Schardl, CL, Siegel, MR (1994) Sexual compatibility and taxonomy of a new species of Epichloë symbiotic with fine fescue grasses. Mycologia 86: 802–812
Mantel, N (1967) The detection of disease clustering and a generalized regression approach. Cancer Res 27: 209–220
Maynard Smith, J, Smith, NH, O'Rourke, M, Spratt, BG (1993) How clonal are bacteria? Proc Natl Acad Sci USA 90: 4384–4388
Meijer, G, Leuchtmann, A (1999) Multistrain infections of the grass Brachypodium sylvaticum by its fungal endophyte Epichloë sylvatica. New Phytol 141: 355–368
Meijer, G, Leuchtmann, A (2001) Fungal genotype controls mutualism and sex in Brachypodium sylvaticum infected by Epichloë sylvatica. Acta Biol Hung 52: 249–263
Milgroom, MG (1996) Recombination and the multi-locus structure of fungal populations. Annu Rev Phytopathol 96: 10518–10523
Moon, CD, Tapper, BA, Scott, B (1999) Identification of Epichloë endophytes in plants by a microsatellite-based PCR fingerprinting assay with automated analysis. Appl Environ Microb 65: 1268–1279
Moon, CD, Craven, KD, Leuchtmann, A, Clements, SL, Schardl, CL (2004) Prevalence of interspecific hybrids amongst asexual fungal endophytes of grasses. Mol Ecol 13: 1455–1467
Moy, M, Belanger, F, Duncan, R, Freehoff, A, Leary, C, Meyer, W, Sullivan, R, White, JF Jr (2000) Identification of epiphyllous mycelial nets on leaves of grasses infected by Clavicipitaceous endophytes. Symbiosis 28: 291–302
Saha, DC, Johnson-Cicalese, JM, Halisky, PM, van Heemstra, MI, Funk, CR (1987) Occurrence and significance of endophytic fungi in the fine fescues. Plant Dis 71:1021–1024
Saha, DC, Jackson, MA, Johnson-Cicalese, JM (1988) A rapid staining method for detection of endophytic fungi in turf and forage grasses. Phytopathol 78: 237–239
Saikkonen, K, Ahlholm, J, Helander, M, Lehtimäki, S, Niemeläinen, O (2000) Endophytic fungi in wild and cultivated grasses in Finland. Ecography 23: 360–366
Saikkonen, K, Faeth, SH, Helander, M, Sullivan, TJ (1998) Fungal endophytes: a continuum of interactions with host plants. Annu Rev Ecol Syst 29: 319–343
Saikkonen, K, Helander, M, Faeth, SH (2004) Fungal endophytes: hitchhikers of the green world. In: Gillings, M, Holmes, A (Eds.) Plant Microbiology. BIOS Scientific Publishers Ltd., Oxford, pp 77–95
Saikkonen, K, Wäli, P, Helander, M, Faeth, SH (2004) Evolution of endophyte–plant symbioses. Trends Plant Sci 9: 275–280
Sampson, K (1933) The systemic infection of grasses by Epichloë typhina (Pers.) Tul. Trans Br Mycol Soc 18: 30–47
Schardl, CL (2001) Epichloë festucae and related mutualistic symbionts of grasses. Fungal Genet Biol 33: 69–82
Schardl, CL, Craven, KD (2003) Interspecific hybridization in plant-associated fungi and oomycetes: a review. Mol Ecol 12: 2861–2873
Schardl, CL, Leuchtmann, A, Spiering, MJ (2004) Symbiosis of grasses with seedborne fungal endophytes. Annu Rev Plant Biol 55: 315–340
Schneider, S, Roessli, D, Excoffier, L (2000) ARLEQUIN ver. 2.00: A software for population genetic data analysis. Genetics and Biometry Laboratory, Dept. of Anthropology and Ecology, University of Geneva, Switzerland
Sullivan, TJ, Faeth, SH (2004) Gene flow in the endophyte Neotyphodium and implications for coevolution with Festuca arizonica. Mol Ecol 13: 649–656
Talbot, NJ, Salch, YP, Ma, M, Hamer, JE (1993) Karyotypic variation within clonal lineages of the rice blast fungus, Magnaporthe grisea. Appl Environ Microb 59: 585–593
Taylor, JW, Jacobson, DJ, Fisher, MC (1999) The evolution of asexual fungi: reproduction, speciation and classification. Annu Rev Phytopathol 37: 197–246
Thompson, JN (1999) The raw material for coevolution. Oikos 84: 5–16
Vandenkoornhuyse, P, Leyval, C, Bonnin, I (2001) High genetic diversity in arbuscular mycorrhizal fungi: evidence for recombination events. Heredity 87: 243–253
Väre, H, Itämies, J (1995) Phorbia phrenione (Seguy) (Diptera: Anthomyiidae) in Finland. Sahlbergia 2: 119–124
Western, JH, Cavett, JJ (1959) The choke disease of cocksfoot (Dactylis glomerata) caused by Epichloë typhina (Fr.) Tul. Trans Br Mycol Soc 42: 298–307
White, JF, Morgan-Jones, G, Morrow, AC (1993) Taxonomy, life cycle, reproduction and detection of Acremonium endophytes. Agricult Ecosyst Environ 44: 13–37
White, JF, Lewis, G, Sun, S, Funk, CR Jr (1993) A study of distribution of Agremonium in populations of red fescue in southwest England and in vitro comparisons to isolates from North American collections. Sydowia 45: 388–394
White, TJ, Burns, T, Lee, S, Taylor, J (1990) Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis, MA, Gelfand, DH, Sninsky, JJ, White TJ (Eds.) PCR Protocols: A Guide to Methods and Amplifications. Academic Press, San Diego, pp 315–322
Wille, PA, Aeschbacher, RA, Boller, T (1999) Distribution of fungal endophyte genotypes in doubly infected host grasses. Plant J 18: 349–358
Wrigth, S (1969) Evolution and the Genetics of Populations: A Treatise in Three Volumes. Vol. 2: The Theory of Gene Frequencies. University of Chicago Press
Zabalgogeazcoa, I, Vázquez de Aldana, BR, García Criado, B, García Ciudad, A (1999) The infection of Festuca rubra by the fungal endophyte Epichloë festucae in permanent Mediterranean grassland. Grass For Sci 54: 91–94
Acknowledgments
Dr. Pia Mutikainen and anonymous reviewers provided valuable comments on the manuscript. We thank Mr. Ilkka Blomqvist for preparing the maps. This study was funded by Academy of Finland and by Finnish Cultural Foundation.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Table 1
Location, habitat endophyte infection frequency and sample size of Festuca rubra populations (PDF 78 kb)
Rights and permissions
About this article
Cite this article
Wäli, P.R., Ahlholm, J.U., Helander, M. et al. Occurrence and Genetic Structure of the Systemic Grass Endophyte Epichloë festucae in Fine Fescue Populations. Microb Ecol 53, 20–29 (2007). https://doi.org/10.1007/s00248-006-9076-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00248-006-9076-2