Abstract
Background
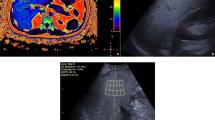
Nonalcoholic fatty liver disease (NAFLD) is the most common cause of chronic liver disease in children. To avoid limitations of liver biopsy and MRI, quantitative ultrasound has become a research focus. Ultrasound-derived fat fraction (UDFF) is based on a combination of backscatter coefficient and attenuation parameter.
Objective
The objectives of the study were to determine (1) agreement between UDFF/MRI proton density fat fraction (MR-PDFF) and (2) whether BMI and age are predictive for UDFF.
Materials and methods
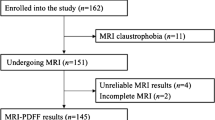
This cross-sectional prospective study included a convenience sample of 46 children referred for clinically indicated abdominal MRI. MR-PDFF and five acquisitions of UDFF were collected. Intraclass correlation coefficient (ICC) and Bland-Altman analysis were used to assess agreement between MR-PDFF and UDFF. Receiver operating characteristic curves were calculated for UDFF prediction of liver steatosis (MR-PDFF ≥ 6%). Multivariable regression was performed to assess BMI and age as predictors for UDFF.
Results
Twenty-two participants were male, 24 were female, and the mean age was 14 ± 3 (range: 7-18) years. Thirty-six out of 46 participants had normal liver fat fraction <6%, and 10/46 had liver steatosis. UDFF was positively associated with MR-PDFF (ICC 0.92 (95% CI, 0.89-0.96). The mean bias between UDFF and MR-PDFF was 0.64% (95% LOA, -5.3-6.6%). AUROC of UDFF for steatosis was of 0.95 (95% CI, 0.89-0.99). UDFF cutoff of 6% had a sensitivity of 90% (95% CI, 55-99%) and a specificity of 94% (95% CI, 81-0.99%). BMI was an independent predictor of UDFF (correlation: 0.55 (95% CI, 0.35-0.95)).
Conclusions
UDFF shows strong agreement with MR-PDFF in children. A UDFF cutoff of 6% provides good sensitivity and specificity for detection of MR-PDFF of ≥ 6%.








Similar content being viewed by others
Data availability
The data that support the findings of this study are not openly available due to reasons of individuals’ privacy and are available from the corresponding author upon reasonable request. Data are located in controlled access data storage at Stanford University.
References
Vos MB, Abrams SH, Barlow SE et al (2017) NASPGHAN clinical practice guideline for the diagnosis and treatment of nonalcoholic fatty liver disease in children: recommendations from the Expert Committee on NAFLD (ECON) and the North American Society of Pediatric Gastroenterology, Hepatology and Nutrition (NASPGHAN). J Pediatr Gastroenterol Nutr 64:319–334. https://doi.org/10.1097/MPG.0000000000001482
Nobili V, Alisi A, Valenti L et al (2019) NAFLD in children: new genes, new diagnostic modalities and new drugs. Nat Rev Gastroenterol Hepatol 16:517–530. https://doi.org/10.1038/s41575-019-0169-z
Anderson EL, Howe LD, Jones HE et al (2015) The prevalence of non-alcoholic fatty liver disease in children and adolescents: a systematic review and meta-analysis. Plos One 10:e0140908. https://doi.org/10.1371/journal.pone.0140908
Boyd A, Cain O, Chauhan A, Webb GJ (2020) Medical liver biopsy: background, indications, procedure and histopathology. Frontline Gastroenterol 11:40–47. https://doi.org/10.1136/flgastro-2018-101139
Nalbantoglu Ilk, Brunt EM Role of liver biopsy in nonalcoholic fatty liver disease. 20:13
Ratziu V, Charlotte F, Heurtier A et al (2005) Sampling variability of liver biopsy in nonalcoholic fatty liver disease. Gastroenterology 128:1898–1906. https://doi.org/10.1053/j.gastro.2005.03.084
Reeder SB, Sirlin CB (2010) Quantification of liver fat with magnetic resonance imaging. Magn Reson Imaging Clin 18:337–357. https://doi.org/10.1016/j.mric.2010.08.013
Noureddin M, Lam J, Peterson MR et al (2013) Utility of magnetic resonance imaging versus histology for quantifying changes in liver fat in nonalcoholic fatty liver disease trials: Hepatology. Hepatology 58:1930–1940. https://doi.org/10.1002/hep.26455
Webb A, Obungoloch J (2023) Five steps to make MRI scanners more affordable to the world. Nature 615:391–393. https://doi.org/10.1038/d41586-023-00759-x
Dasarathy S, Dasarathy J, Khiyami A et al (2009) Validity of real time ultrasound in the diagnosis of hepatic steatosis: a prospective study. J Hepatol 51:1061–1067. https://doi.org/10.1016/j.jhep.2009.09.001
Strauss S, Gavish E, Gottlieb P, Katsnelson L (2007) Interobserver and intraobserver variability in the sonographic assessment of fatty liver. Am J Roentgenol 189:W320–W323. https://doi.org/10.2214/AJR.07.2123
Bohte AE, Koot BGP, van der Baan-Slootweg OH et al (2012) US cannot be used to predict the presence or severity of hepatic steatosis in severely obese adolescents. Radiology 262:327–334. https://doi.org/10.1148/radiol.11111094
Diagnostic Radiology, Faculty of Medicine, Chulalongkorn University, Bangkok, Thailand, Tanpowpong N, Panichyawat S, Diagnostic Radiology, Faculty of Medicine, Chulalongkorn University, Bangkok, Thailand (2020) Comparison of sonographic hepatorenal ratio and the degree of hepatic steatosis in magnetic resonance imaging-proton density fat fraction. J Ultrason 20:169–175. https://doi.org/10.15557/JoU.2020.0028
Pacifico L, Celestre M, Anania C et al (2007) MRI and ultrasound for hepatic fat quantification: relationships to clinical and metabolic characteristics of pediatric nonalcoholic fatty liver disease. Acta Paediatrica 96:542–547. https://doi.org/10.1111/j.1651-2227.2007.00186.x
Nam K, Zagzebski JA, Hall TJ (2011) Simultaneous backscatter and attenuation estimation using a least squares method with constraints. Ultrasound in Med Biol 37:2096–2104. https://doi.org/10.1016/j.ultrasmedbio.2011.08.008
Runge JH, van Giessen J, Draijer LG et al (2021) Accuracy of controlled attenuation parameter compared with ultrasound for detecting hepatic steatosis in children with severe obesity. Eur Radiol 31:1588–1596. https://doi.org/10.1007/s00330-020-07245-2
Ferraioli G, Maiocchi L, Savietto G et al (2021) Performance of the attenuation imaging technology in the detection of liver steatosis. J Ultrasound Med 40:1325–1332. https://doi.org/10.1002/jum.15512
Cailloce R, Tavernier E, Brunereau L et al (2021) Liver shear wave elastography and attenuation imaging coefficient measures: prospective evaluation in healthy children. Abdom Radiol 46:4629–4636. https://doi.org/10.1007/s00261-021-02960-w
Labyed Y, Milkowski A (2020) Novel method for ultrasound-derived fat fraction using an integrated phantom. J Ultrasound Med 39:2427–2438. https://doi.org/10.1002/jum.15364
Dillman JR, Thapaliya S, Tkach JA, Trout AT (2022) Quantification of hepatic steatosis by ultrasound: prospective comparison with MRI proton density fat fraction as reference standard. Am J Roentgenol 219:784–791. https://doi.org/10.2214/AJR.22.27878
Tang A, Desai A, Hamilton G et al (2015) Accuracy of MR imaging–estimated proton density fat fraction for classification of dichotomized histologic steatosis grades in nonalcoholic fatty liver disease. Radiology 274:416–425. https://doi.org/10.1148/radiol.14140754
Lin SC, Heba E, Wolfson T et al (2015) Noninvasive diagnosis of nonalcoholic fatty liver disease and quantification of liver fat using a new quantitative ultrasound technique. Clin Gastroenterol Hepatol 13:1337-1345.e6. https://doi.org/10.1016/j.cgh.2014.11.027
Han A, Zhang YN, Boehringer AS et al (2020) Assessment of hepatic steatosis in nonalcoholic fatty liver disease by using quantitative US. Radiology 295:106–113. https://doi.org/10.1148/radiol.2020191152
Sasso M, Beaugrand M, de Ledinghen V et al (2010) Controlled attenuation parameter (CAP): a novel VCTETM guided ultrasonic attenuation measurement for the evaluation of hepatic steatosis: preliminary study and validation in a cohort of patients with chronic liver disease from various causes. Ultrasound Med Biol 36:1825–1835. https://doi.org/10.1016/j.ultrasmedbio.2010.07.005
D’Hondt A, Rubesova E, Xie H et al (2021) Liver fat quantification by ultrasound in children: a prospective study. Am J Roentgenol 217:996–1006. https://doi.org/10.2214/AJR.20.24874
Webb M, Yeshua H, Zelber-Sagi S et al (2009) Diagnostic value of a computerized hepatorenal index for sonographic quantification of liver steatosis. Am J Roentgenol 192:909–914. https://doi.org/10.2214/AJR.07.4016
Marshall RH, Eissa M, Bluth EI et al (2012) Hepatorenal index as an accurate, simple, and effective tool in screening for steatosis. Am J Roentgenol 199:997–1002. https://doi.org/10.2214/AJR.11.6677
Frankland MP, Dillman JR, Anton CG et al (2022) Diagnostic performance of ultrasound hepatorenal index for the diagnosis of hepatic steatosis in children. Pediatric Radiology 52:1306–1313. https://doi.org/10.1007/s00247-022-05313-x
Ferraioli G, Monteiro LBS (2019) Ultrasound-based techniques for the diagnosis of liver steatosis. WJG 25:6053–6062. https://doi.org/10.3748/wjg.v25.i40.6053
Ferraioli G, Maiocchi L, Raciti MV, et al (2019) Detection of liver steatosis with a novel ultrasound-based technique: a pilot study using MRI-derived proton density fat fraction as the gold standard. Clinical and Translational Gastroenterology 10:e00081. https://doi.org/10.14309/ctg.0000000000000081
Qu Y, Li M, Hamilton G et al (2019) Diagnostic accuracy of hepatic proton density fat fraction measured by magnetic resonance imaging for the evaluation of liver steatosis with histology as reference standard: a meta-analysis. Eur Radiol 29:5180–5189. https://doi.org/10.1007/s00330-019-06071-5
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the Ethics Committee of Stanford University (Date: 30/06/2020/No. IRB-46543).
Conflicts of interest
Max Zalcman has received partial financial research support from the Belgian American Educational Foundation (B.A.E.F.) to conduct this study. No funds, grants, or other support was received by Richard Barth and Erika Rubesova. The authors declare they have no financial interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zalcman, M., Barth, R.A. & Rubesova, E. Real-time ultrasound-derived fat fraction in pediatric population: feasibility validation with MR-PDFF. Pediatr Radiol 53, 2466–2475 (2023). https://doi.org/10.1007/s00247-023-05752-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00247-023-05752-0