Abstract
Background
Medulloblastoma, a high-grade embryonal tumor, is the most common primary brain malignancy in the pediatric population. Molecular medulloblastoma groups have documented clinically and biologically relevant characteristics. Several authors have attempted to differentiate medulloblastoma molecular groups and histology variants using diffusion-weighted imaging (DWI) and apparent diffusion coefficient (ADC) maps. However, literature on the use of ADC histogram analysis in medulloblastomas is still scarce.
Objective
This study presents data from a sizable group of pediatric patients with medulloblastoma from a single institution to determine the performance of ADC histogram metrics for differentiating medulloblastoma variants and groups based on both histological and molecular features.
Materials and methods
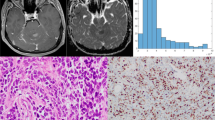
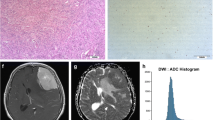
In this retrospective study, we evaluated the distribution of absolute and normalized ADC values of medulloblastomas. Tumors were manually segmented and diffusivity metrics calculated on a pixel-by-pixel basis. We calculated a variety of first-order histogram metrics from the ADC maps, including entropy, minimum, 10th percentile, 90th percentile, maximum, mean, median, skewness and kurtosis, to differentiate molecular and histological variants. ADC values of the tumors were also normalized to the bilateral cerebellar cortex and thalami. We used the Kruskal–Wallis and Mann–Whitney U tests to evaluate differences between the groups. We carried out receiver operating characteristic (ROC) curve analysis to evaluate the areas under the curves and to determine the cut-off values for differentiating tumor groups.
Results
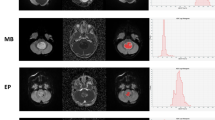
We found 65 children with confirmed histopathological diagnosis of medulloblastoma. Mean age was 8.3 ± 5.8 years, and 60% (n = 39) were male. One child was excluded because histopathological variant could not be determined. In terms of medulloblastoma variants, tumors were classified as classic (n = 47), desmoplastic/nodular (n = 9), large/cell anaplastic (n = 6) or as having extensive nodularity (n = 2). Seven other children were excluded from the study because of incomplete imaging or equivocal molecular diagnosis. Regarding medulloblastoma molecular groups, there were: wingless (WNT) group (n = 7), sonic hedgehog (SHH) group (n = 14) and non-WNT/non-SHH (n = 36). Our results showed significant differences among the molecular groups in terms of the median (P = 0.002), mean (P = 0.003) and 90th percentile (P = 0.002) ADC histogram metrics. No significant differences among the various medulloblastoma histological variants were found.
Conclusion
ADC histogram analysis can be implemented as a complementary tool in the preoperative evaluation of medulloblastoma in children. This technique can provide valuable information for differentiating among medulloblastoma molecular groups. ADC histogram metrics can help predict medulloblastoma molecular classification preoperatively.








Similar content being viewed by others
References
Crawford JR, MacDonald TJ, Packer RJ (2007) Medulloblastoma in childhood: new biological advances. Lancet Neurol 6:1073–1085
MacDonald TJ (2008) Aggressive infantile embryonal tumors. J Child Neurol 23:1195–1204
Smoll NR, Drummond KJ (2012) The incidence of medulloblastomas and primitive neurectodermal tumours in adults and children. J Clin Neurosci 19:1541–1544
Battaglia S, Albini Riccioli L, Zucchelli M, Leonardi M (2007) 3 tesla MR study of cerebellar medulloblastoma. Neuroradiol J 19:722–726
Frič R, Due-Tønnessen BJ, Lundar T et al (2020) Long-term outcome of posterior fossa medulloblastoma in patients surviving more than 20 years following primary treatment in childhood. Sci Rep 10:9371
Thompson MC, Fuller C, Hogg TL et al (2006) Genomics identifies medulloblastoma subgroups that are enriched for specific genetic alterations. J Clin Oncol 24:1924–1931
Cho Y-J, Tsherniak A, Tamayo P et al (2011) Integrative genomic analysis of medulloblastoma identifies a molecular subgroup that drives poor clinical outcome. J Clin Oncol 29:1424–1430
Kool M, Koster J, Bunt J et al (2008) Integrated genomics identifies five medulloblastoma subtypes with distinct genetic profiles, pathway signatures and clinicopathological features. PLoS One 3:e3088
Jones DTW, Jäger N, Kool M et al (2012) Dissecting the genomic complexity underlying medulloblastoma. Nature 488:100–105
Northcott PA, Shih DJH, Peacock J et al (2012) Subgroup-specific structural variation across 1,000 medulloblastoma genomes. Nature 488:49–56
Pugh TJ, Weeraratne SD, Archer TC et al (2012) Medulloblastoma exome sequencing uncovers subtype-specific somatic mutations. Nature 488:106–110
Robinson G, Parker M, Kranenburg TA et al (2012) Novel mutations target distinct subgroups of medulloblastoma. Nature 488:43–48
Pomeroy SL, Tamayo P, Gaasenbeek M et al (2002) Prediction of central nervous system embryonal tumour outcome based on gene expression. Nature 415:436–442
Northcott PA, Korshunov A, Witt H et al (2011) Medulloblastoma comprises four distinct molecular variants. J Clin Oncol 29:1408–1414
Louis DN, Perry A, Wesseling P et al (2021) The 2021 WHO classification of tumors of the central nervous system: a summary. Neuro Oncol 23:1231–1251
Orr BA (2020) Pathology, diagnostics, and classification of medulloblastoma. Brain Pathol 30:664–678
Taylor MD, Northcott PA, Korshunov A et al (2012) Molecular subgroups of medulloblastoma: the current consensus. Acta Neuropathol 123:465–472
Menyhárt O, Giangaspero F, Győrffy B (2019) Molecular markers and potential therapeutic targets in non-WNT/non-SHH (group 3 and group 4) medulloblastomas. J Hematol Oncol 12:29
Juraschka K, Taylor MD (2019) Medulloblastoma in the age of molecular subgroups: a review. J Neurosurg Pediatr 24:353–363
Perreault S, Ramaswamy V, Achrol AS et al (2014) MRI surrogates for molecular subgroups of medulloblastoma. AJNR Am J Neuroradiol 35:1263–1269
Reddy N, Ellison DW, Soares BP et al Pediatric posterior fossa medulloblastoma: the role of diffusion imaging in identifying molecular groups. J Neuroimaging 30:503–511
Reis J, Stahl R, Zimmermann H et al (2021) Advanced MRI findings in medulloblastomas: relationship to genetic subtypes, histopathology, and immunohistochemistry. J Neuroimaging 31:306–316
Al-Sharydah AM, Al-Arfaj HK, Saleh Al-Muhaish H et al (2019) Can apparent diffusion coefficient values help distinguish between different types of pediatric brain tumors? Eur J Radiol Open 6:49–55
Payabvash S, Tihan T, Cha S (2018) Volumetric voxelwise apparent diffusion coefficient histogram analysis for differentiation of the fourth ventricular tumors. Neuroradiol J 31:554–564
Yamasaki F, Kurisu K, Satoh K et al (2005) Apparent diffusion coefficient of human brain tumors at MR imaging. Radiology 235:985–991
Xianwang L, Lei H, Hong L et al (2020) Apparent diffusion coefficient to evaluate adult intracranial ependymomas: relationship to Ki-67 proliferation index. J Neuroimaging 31:132–136
Schob S, Beeskow A, Dieckow J et al (2018) Diffusion profiling of tumor volumes using a histogram approach can predict proliferation and further microarchitectural features in medulloblastoma. Childs Nerv Syst 34:1651–1656
Schob S, Meyer HJ, Dieckow J et al (2017) Histogram analysis of diffusion weighted imaging at 3T is useful for prediction of lymphatic metastatic spread, proliferative activity, and cellularity in thyroid cancer. Int J Mol Sci 18:821
Schob S, Meyer J, Gawlitza M et al (2016) Diffusion-weighted MRI reflects proliferative activity in primary CNS lymphoma. PLoS One 11:e0161386
Schob S, Meyer HJ, Pazaitis N et al (2017) ADC histogram analysis of cervical cancer aids detecting lymphatic metastases — a preliminary study. Mol Imaging Biol 19:953–962
Khrichenko D (n.d.) pMRI. Children’s Hospital of Philadelphia. https://www.parametricmri.com. Accessed 23 Apr 2022
Ogura A, Tamura T, Ozaki M et al (2015) Apparent diffusion coefficient value is not dependent on magnetic resonance systems and field strength under fixed imaging parameters in brain. J Comput Assist Tomogr 39:760–765
de Souza Cassia G, Alves CAPF, Taranath A et al (2018) Childhood medulloblastoma revisited. Top Magn Reson Imaging 27:479–502
Yeom KW, Mobley BC, Lober RM et al (2013) Distinctive MRI features of pediatric medulloblastoma subtypes. AJR Am J Roentgenol 200:895–903
Fruehwald-Pallamar J, Puchner SB, Rossi A et al (2011) Magnetic resonance imaging spectrum of medulloblastoma. Neuroradiology 53:387–396
Teo W-Y, Shen J, Su JMF et al (2013) Implications of tumor location on subtypes of medulloblastoma. Pediatr Blood Cancer 60:1408–1410
Robinson GW (2013) Impact of tumor location on medulloblastoma subtyping and treatment. Pediatr Blood Cancer 60:1393–1394
Wefers AK, Warmuth-Metz M, Pöschl J et al (2014) Subgroup-specific localization of human medulloblastoma based on pre-operative MRI. Acta Neuropathol 127:931–933
Neumann JE, Swartling FJ, Schüller U (2017) Medulloblastoma: experimental models and reality. Acta Neuropathol 134:679–689
Kovanlikaya A, Panigrahy A, Krieger MD et al (2005) Untreated pediatric primitive neuroectodermal tumor in vivo: quantitation of taurine with MR spectroscopy. Radiology 236:1020–1025
Panigrahy A, Krieger MD, Gonzalez-Gomez I et al (2006) Quantitative short echo time 1H-MR spectroscopy of untreated pediatric brain tumors: preoperative diagnosis and characterization. AJNR Am J Neuroradiol 27:560–572
Blüml S, Margol AS, Sposto R et al (2016) Molecular subgroups of medulloblastoma identification using noninvasive magnetic resonance spectroscopy. Neuro Oncol 18:126–131
Dangouloff-Ros V, Varlet P, Levy R et al (2021) Imaging features of medulloblastoma: conventional imaging, diffusion-weighted imaging, perfusion-weighted imaging, and spectroscopy: from general features to subtypes and characteristics. Neurochirurgie 67:6–13
Rumboldt Z, Camacho DLA, Lake D et al (2006) Apparent diffusion coefficients for differentiation of cerebellar tumors in children. AJNR Am J Neuroradiol 27:1362–1369
Pierce TT, Provenzale JM (2014) Evaluation of apparent diffusion coefficient thresholds for diagnosis of medulloblastoma using diffusion-weighted imaging. Neuroradiol J 27:63–74
Thompson EM, Hielscher T, Bouffet E et al (2016) Prognostic value of medulloblastoma extent of resection after accounting for molecular subgroup: a retrospective integrated clinical and molecular analysis. Lancet Oncol 17:484–495
Sutton LN, Phillips PC, Molloy PT (1996) Surgical management of medulloblastoma. J Neurooncol 29:9–21
Pols SYCV, van Veelen MLC, Aarsen FK et al (2017) Risk factors for development of postoperative cerebellar mutism syndrome in children after medulloblastoma surgery. J Neurosurg Pediatr 20:35–41
Yan J, Liu L, Wang W et al (2020) Radiomic features from multi-parameter MRI combined with clinical parameters predict molecular subgroups in patients with medulloblastoma. Front Oncol 10:558162
Acknowledgments
The authors would like to acknowledge Lydia Sheldon for editing and proofreading the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
None
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Gonçalves, F.G., Tierradentro-Garcia, L.O., Kim, J.D.U. et al. The role of apparent diffusion coefficient histogram metrics for differentiating pediatric medulloblastoma histological variants and molecular groups. Pediatr Radiol 52, 2595–2609 (2022). https://doi.org/10.1007/s00247-022-05411-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00247-022-05411-w