Abstract
Background
Radiation dose metrics vary by the calibration reference phantom used to report doses. By convention, 16-cm diameter cylindrical polymethyl-methacyrlate phantoms are used for head imaging and 32-cm diameter phantoms are used for body imaging in adults. Actual usage patterns in children remain under-documented.
Objective
This study uses the University of California San Francisco International CT Dose Registry to describe phantom selection in children by patient age, body region and scanner manufacturer, and the consequent impact on radiation doses.
Materials and methods
For 106,837 pediatric computed tomography (CT) exams collected between Jan. 1, 2015, and Nov. 2, 2020, in children up to 17 years of age from 118 hospitals and imaging facilities, we describe reference phantom use patterns by body region, age and manufacturer, and median and 75th-percentile dose–length product (DLP) and volume CT dose index (CTDIvol) doses when using 16-cm vs. 32-cm phantoms.
Results
There was relatively consistent phantom selection by body region. Overall, 98.0% of brain and skull examinations referenced 16-cm phantoms, and 95.7% of chest, 94.4% of abdomen and 100% of cervical-spine examinations referenced 32-cm phantoms. Only GE deviated from this practice, reporting chest and abdomen scans using 16-cm phantoms with some frequency in children up to 10 years of age. DLP and CTDIvol values from 16-cm phantom-referenced scans were 2–3 times higher than 32-cm phantom-referenced scans.
Conclusion
Reference phantom selection is highly consistent, with a small but significant number of abdomen and chest scans (~5%) using 16-cm phantoms in younger children, which produces DLP values approximately twice as high as exams referenced to 32-cm phantoms
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The rapid rise over the last few decades in computed tomography (CT) imaging and consequent population exposure to ionizing radiation, a known carcinogen, have raised concerns about the levels and variability of radiation doses across patients, institutions and countries, as well as the need for dose optimization [1,2,3,4,5,6,7,8]. Diverse organizations and campaigns, such as Choosing Wisely and Image Gently, promote improving the safety and effective imaging care of children worldwide to optimize and reduce patient radiation dose exposures [9, 10].
Dose optimization tools like diagnostic reference levels use metrics such as the volume CT dose index (CTDIvol), reflecting the average dose (per slice) over the total volume scanned for the selected CT conditions of operation, and the dose–length product (DLP), reflecting the total dose imparted to the patient. While these metrics reflect scanner output and not patient absorbed dose, they correlate closely with absorbed doses and help physicians and imaging practices compare their doses to a uniform standard [11].
CTDIvol values are reported directly from the scanner and must be referenced to a calibration reference phantom for reporting. By convention, 16-cm diameter cylindrical polymethyl-methacyrlate phantoms are used for head imaging and 32-cm diameter phantoms are used for body imaging in adults. Accuracy (validity) of the estimated dose to reflect the true patient absorbed dose depends on the closeness of fit between the volumes of the imaged body section and the reference phantom, as well as kilovoltage peak (kVp) setting and bow-tie filter. The 32-cm body phantom corresponds to a patient with a 47-in. (~120 cm) waistline. Therefore, a dose estimate for very small patients based on a 32-cm phantom at 120 kVp will underestimate the true patient absorbed dose by approximately a factor of 2, and vice versa; a CTDIvol of 8 mGy from a 16-cm phantom vs. a CTDIvol of 4 mGy from a 32-cm phantom would indicate the same CT output [12, 13].
An underappreciated challenge in pediatric dosimetry concerns the choice of phantom for dose reporting, as pediatric phantom selection may be inconsistent [14]. The source of this variation may be that some manufacturers follow adult conventions, while other manufacturers choose the smaller 16-cm phantoms for reporting abdomen and chest doses in children, as this more closely reflects actual patient size [15, 16]. The reported dose will vary considerably between the two phantom sizes, even when the technical parameters are identical [17]. This inconsistency in reporting can result in patient distress and confusion when they undergo scans on machines with different reporting conventions [18].
Several investigators have created ad hoc corrections, for example suggesting that CTDIvol and DLP values estimated from 32-cm diameter phantoms should be multiplied by a factor of 2 to obtain “correct” values in pediatric body scans [19]. This problem is not only important when understanding an individual patient’s dose, but also when trying to optimize protocols because the applicability of a benchmark will vary depending on what phantom was used. Some pediatric reference values have been explicitly reported using only one specific size reference phantom, but unless dose comparisons use the same size phantom, it is easy to unknowingly introduce errors [20]. Similarly, the Alliance for Radiation Safety in Pediatric Imaging created conversion factors for normalizing CTDIvol and DLP to patient size to estimate actual absorbed doses and specified that these values be consistently calculated with the 32-cm phantom [21].
Despite recognition of the importance of phantom selection in pediatric dosimetry, we lack representative data on what phantoms are used in actual practice, how these selections vary by manufacturer, and how the reported doses vary by phantom size in actual practice. Using data from a large multicenter CT dose registry, this study describes variations in practice and differences in estimated doses that result from the differential use of 16-cm (head) and 32-cm (body) phantoms in young patients.
Materials and methods
Registry
The University of California San Francisco (UCSF) International CT Dose Registry includes 6.65 million CT exams assembled from across 160 hospital and imaging facilities [6, 7]. The registry was created with funding from the University of California Office of the President, the Centers for Disease Control and Prevention, the National Institutes of Health and the Patient Centered Outcomes Research Institute, and includes data from health care institutions that used Radimetrics Radiation Dose Management Solution (Bayer HealthCare, Whippany, NJ) and expressed interest in collaborating with UCSF on radiation-related research. The UCSF Institutional Review Board approved the registry study and waived informed consent. Collaborating institutions either approved the study locally or relied on UCSF approval.
Study population
We included 106,837 pediatric diagnostic CT examinations obtained in 118 U.S. facilities for children under 18 years of age performed between Jan. 1, 2015, and Nov. 2, 2020, that included imaging of the head, cervical spine (c-spine), chest, or abdomen and pelvis (abdomen). We divided head scans into brain and skull imaging (including sinus, facial bones and temporal bones); neck and c-spine exams are included in a single category. These body regions reflect 87% of all exams during the study period. We excluded CTs that included insufficient numbers for analysis or that covered multiple body parts (n=15,849 or 13% of all scans), or those performed as part of radiation oncology guidance, surgical or interventional procedures, combined positron emission tomography (PET)-CT and single photon emission CT (SPECT) imaging.
Manufacturers
We included scans from four manufacturers: Canon Medical Systems Corporation (including Toshiba; Ōtawara, Tochigi, Japan), GE Healthcare (Chicago, Illinois), Philips (Koninklijke Philips N.V., Amsterdam, The Netherlands) and Siemens (Siemens Healthcare, Erlangen, Germany). The sample includes 41 unique scanner models (Canon/Toshiba: 5, GE: 18, Philips: 6, Siemens: 12) and 247 individual scanners.
Variables
We report DLP, which reflects the total scanner emitted radiation, defined as the product of CTDIvol and the scan length, reflecting the total radiation output received by the patient for a CT scan and measured in mGy·cm. Each dose metric is referenced to a 16-cm or 32-cm phantom. Results are shown for complete CT examinations including all irradiating events (excluding scouts, localizers and boluses). A CT examination including a scan with and a scan without contrast is considered a single examination. Exam-level DLP is calculated as the sum of all constituent series-level DLP values. For simplicity, we excluded multiphase examinations that were referenced to more than one phantom (n=7,204). We categorized patients into the five mutually exclusive age groups used by the Leapfrog Group [22]: <1 year, 1–4 years, 5–9 years, 10–14 years, 15–17 years.
Statistical analysis
For each body region, we report the number and percent of examinations that used 16-cm and 32-cm phantoms, stratified by body region, patient age and manufacturer. We calculated the number and proportion of exams using the “expected” phantom, based on predominate usage patterns across all manufacturers (16 cm for brain and skull, 32 cm for chest, abdomen and c-spine) by body region, age group and scanner manufacturer.
We calculated the median and 75th percentiles for each dose metric, stratified by body region, patient age and manufacturer, and calculated the relative median dose (i.e. ratio) between phantom sizes (16 cm vs. 32 cm) to measure the magnitude of difference due to reference phantom selection when there were at least 5 CT examinations performed by age and body region using each phantom. The Radimetrics dose tracking platform was employed to extract all patient, scanner and exam variables (see [6, 7] for details), and SAS (version 9.3; SAS Institute, Cary, NC) and R (version 3.6.3; R Foundation for Statistical Computing, Vienna, Austria) were used for all analyses.
Results
Overall, 54.6% of the exams were comprised of males, 59.2% of exams used 16-cm phantoms and the most common body region imaged was the head, including the brain (n=48,680, 45.6%) and skull (n=12,929, 12.1%). A total of 44.1% of exams were performed at pediatric-specific hospitals (Table 1). Across all body regions, the number of scans generally increased with age (Table 2).
Phantom selection varied by body region, and for most patients the phantom choice was the same as in adults (Table 2). The 16-cm phantom was used in more than 98.0% of examinations for brain and skull CT examinations regardless of patient age. The 32-cm phantom was used for most chest examinations (95.7%) and abdomen examinations (94.4%), and use of the 32-cm phantom increased with increasing patient age. The 32-cm phantom was used for 100% of c-spine CT examinations.
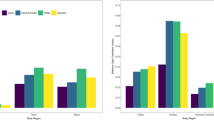
We observed consistent use of the 16-cm phantom for brain and skull imaging across manufacturers with few exceptions, while greater differences in phantom selection by manufacturer were observed for chest and abdomen CT examinations (Table 3). Philips and Siemens used the 32-cm phantom in more than 99% of children for both chest and abdomen CT. For chest CT, Canon used the 32-cm phantom uniformly above age 5, and GE used the 32-cm phantom in more than 95% of children above age 10; in the younger age groups, GE used the 32-cm phantom in 74.7–80.0%. For abdomen CT, GE used the 16-cm phantom frequently in children up to age 10. There is a clear relationship with age in the use of the 32-cm phantom for reporting abdomen and chest CT (Fig. 1). For example, for GE, use of the 32-cm phantom ranges from 45.3% of children <1 year old to 98.5% of children 15–17 years old; for Canon, use of the 32-cm phantom ranges from 92.3% of children <1 year old to 100% of children 15–17 years old.
Most of the CT examinations are reported using a consistent phantom choice. Nonetheless, 0.4% of head scans (n=243) used 32-cm phantoms and 5.2% of chest and abdomen scans (n=1,877) used 16-cm phantoms. The use of 32-cm phantoms for head/skull scans is difficult to explain, though we suspect the use of “body” protocols could play a role. The use of 16-cm phantoms in chest and abdomen scans, on the other hand, could indicate intentional efforts to select a best size match, or manufacturer-specific rules related to scanning parameters such as field of view.
The use of different phantoms has a large impact on reported dose metrics. The median DLP by body region, patient age and manufacturer reported when using each phantom is shown in Table 4. Note that we omitted all c-spine and all Philips combinations from the table because none had the minimum number of five scans of each phantom size to allow comparison. The relative median DLP is approximately twofold higher when using 16-cm phantom (range: 0.7–4.9). While DLP generally increases with advancing age in the pediatric population (not necessarily in adults), these data show that the relative DLP (between 16-cm and 32-cm phantoms) generally declines with advancing age, though inconsistently. For example, the relative dose for chest exams in GE scanners actually increases from 2.8 in patients <1 year old to 4.9 in patients 1–4 years old, before decreasing thereafter for reasons we cannot explain. Results are similar when comparing the 75th percentiles of DLP and relative DLP, with an average 1.9-fold higher dose (range: 0.8–6.0) when reported using the 16-cm phantom (Online Supplementary Material 1).
Table 5 shows the same comparisons for CTDIvol, which partially removes scan length as a confounding factor. In almost all cases, the comparable ratios of relative dose exceed the values for DLP (Table 4). Results for the 75th percentiles of CTDIvol are similar to the medians, with relative doses two- to threefold higher (range: 1.0–5.5) when reported using the 16-cm phantom (Online Supplementary Material 2).
Discussion
Using a large multicenter CT dose registry, we report phantom selection by body region, patient age and scanner manufacturer, and its impact on reported dose. Our findings demonstrate that most scans are reported consistently: 99% of head scans are reported using the 16-cm phantom and 95% of chest and abdomen scans are reported using the 32-cm phantom. Nonetheless, the overall consistency masks notable differences in phantom selection by manufacturer, most notably that GE frequently uses the 16-cm phantom for abdomen CT in younger children. We found, as expected, that the reported DLP values are approximately twice as high when the 16-cm vs. the 32-cm phantom is selected. With growing interest in CT dose documentation, reflected in annual hospital surveys of pediatric doses performed by the Leapfrog Group [22] and regulatory requirements of radiation dose reports in the medical record [23], it is important to use consistent standards across all patients so that physicians, radiology technologists, patients and researchers can clearly and accurately understand the results and know that they were calculated consistently.
While our research highlights both similarities and differences in phantom selection by manufacturer, even consistency in reporting might not reflect best practice. For example, while all manufacturers used the 32-cm phantom for c-spine exams, because of the large difference in size between the 32-cm phantom and child (or adult) neck sizes, reported DLP values for neck scans will markedly underestimate absorbed doses unless some adjustment factor is employed. Similarly, GE frequently uses the 16-cm phantom when reporting chest and abdomen doses in small children — a sensible decision when the 16-cm phantom more closely approximates their size than the 32-cm phantom. Yet this will result in reporting of a significantly higher dose for a given child than had that child been scanned on a device that used the 32-cm phantom. The impact on a patient when they are scanned using devices that report differently can be substantial [19]. Watson and Coakley [14] reported the inconsistent rules that the manufacturers used for selection of the phantom over a decade ago.
There is a robust discussion in the radiology and medical physics literature regarding how to best estimate patient absorbed dose using scanner output combined with information on patient size in order to understand how well scan settings have been tailored to patient size [14]. As an objective way to adjust the CTDIvol to a closer representation of the actual dose delivered to the patient, and hence partially correct for the mismatch between phantom and patient dimensions, size-specific dose estimates (SSDE) were developed [24, 25]. Nonetheless, how accurately reported dose will reflect patient absorbed dose when the phantom is poorly matched to patient size remains an important question because patient doses will be underestimated when 32-cm phantoms are used in smaller patients. This paper does not address this important question, but instead focuses on how the basic interpretation of CT scanner dose output is highly dependent on which phantom is used for reporting. The scanner output must be understood in terms of the actual phantom selected; on average, all else equal, the same DLP dose output will be reported approximately twofold higher if it is scaled to a 16-cm rather than a 32-cm phantom. Our purpose was to demonstrate the magnitude of typical differences in dose that may be obscured by existing pediatric reference value studies and individual clinical applications.
This study has limitations. The sample includes data filtered through a single dose-management software vendor. However, all metrics come directly from either the radiation dose structured report or from the dose report images (via optical character recognition). Consequently, this convenience sample should not affect the phantom-derived dose differences we found. These analyses are limited to 41 scanner models from 4 manufacturers. The current sample size is insufficient to stratify phantom usage patterns by type of facility (e.g., pediatric vs. adult hospital or academic vs. community setting); however, this would be an important and worthwhile area of future study. Ideally, one would stratify and determine optimal pediatric dosing by patient size rather than age, which is a relatively poor predictor of patient diameter [26]. However, actual patient size is usually missing from Digital Imaging and Communications in Medicine data, and we were not able to generate tables by patient size. Similarly, we do not report SSDE values as they are frequently missing in the Radimetrics-derived data, unlike DLP and CTDIvol. In addition, we did not attempt to control for kVp setting, which is known to impact conversion of CTDIvol from 16-cm to 32-cm phantoms. Lastly, this paper did not explore manufacturer rules and algorithms for phantom selection, though this is an important question for future study.
Conclusion
These analyses empirically elucidate reference phantom selection patterns by body region, patient age and scanner manufacturer, and also demonstrate the substantial differences in scanner-reported DLP that arise due to reference phantom selection in clinical studies. Without specifying or stratifying by phantom size, any reporting of aggregate DLP values unwittingly will show a weighted summary that depends on the (unspecified) mixture of scanner manufacturers, patient ages and sizes, and phantoms used. While the use of SSDE avoids some of these problems, standardization of both phantom selection and phantom reporting would improve clinical, research and monitoring applications.
References
National Council on Radiation Protection and Measurements (2009) NCRP Report No. 160, ionizing radiation exposure of the population of the United States. NCRP, Bethesda, MD
National Council on Radiation Protection and Measurements (2019) NCRP Report No. 184, medical radiation exposure of patients in the United States. NCRP, Bethesda, MD
Pearce MS, Salotti JA, Little MP et al (2012) Radiation exposure from CT scans in childhood and subsequent risk of leukaemia and brain tumours: a retrospective cohort study. Lancet 380:499–505
Preston DL, Ron E, Tokuoka S et al (2007) Solid cancer incidence in atomic bomb survivors: 1958–1998. Radiat Res 168:1–64
National Research Council, Committee to Assess Health Risks from Exposure to Low Levels of Ionizing Radiation, Division on Earth and Life Sciences, Board on Radiation Effects Research (2006) Health risks from exposure to low levels of ionizing radiation: BEIR VII phase 2. National Academies Press, Washington, DC
Smith-Bindman R, Wang Y, Chu P et al (2019) International variation in radiation dose for computed tomography examinations: prospective cohort study. BMJ 364:k4931
Smith-Bindman R, Chu P, Wang Y et al (2020) Comparison of the effectiveness of single-component and multicomponent interventions for reducing radiation doses in patients undergoing computed tomography: a randomized clinical trial. JAMA Intern Med 180:666–675
European Commission (2014) Diagnostic reference levels in thirty-six European countries, part 2/2. Publications Office of the European Union, Luxembourg
American Board of Internal Medicine Foundation (2017) Choosing wisely. An initiative of the ABIM foundation. http://www.choosingwisely.org. Accessed 9 Sep 2021
Goske MJ, Applegate KE, Boylan J et al (2008) Image Gently: a national education and communication campaign in radiology using the science of social marketing. J Am Coll Radiol 5:1200–1205
National Council on Radiation Protection and Measurements (2012) NCRP Report No. 172, reference levels and achievable doses in medical and dental imaging: recommendations for the United States. NCRP, Bethesda, MD
Nelson TR (2014) Practical strategies to reduce pediatric CT radiation dose. J Am Coll Radiol 11:292–299
Nickoloff EL, Dutta AK, Lu ZF (2003) Influence of phantom diameter, kVp and scan mode upon computed tomography dose index. Med Phys 30:395–402
Watson DJ, Coakley KS (2010) Paediatric CT reference doses based on weight and CT dosimetry phantom size: local experience using a 64-slice CT scanner. Pediatr Radiol 40:693–703
Fujii K, Aoyama T, Koyama S, Kawaura C (2007) Comparative evaluation of organ and effective doses for paediatric patients with those for adults in chest and abdominal CT examinations. Br J Radiol 80:657–667
Huda W, Vance A (2007) Patient radiation doses from adult and pediatric CT. AJR Am J Roentgenol 188:540–546
Nievelstein RA, van Dam IM, van der Molen AJ (2010) Multidetector CT in children: current concepts and dose reduction strategies. Pediatr Radiol 40:1324–1344
Seibert JA, Boone JM, Wooten-Gorges SL, Lamba R (2014) Dose is not always what it seems: where very misleading values can result from volume CT dose index and dose length product. J Am Coll Radiol 11:233–237
Chapple CL, Broadhead DA, Faulkner K (1995) A phantom based method for deriving typical patient doses from measurements of dose–area product on populations of patients. Br J Radiol 68:1083–1086
Shrimpton PC, Hillier MC, Lewis MA, Dunn M (2006) National survey of doses from CT in the UK: 2003. Br J Radiol 79:968–980
International Electrotechnical Commission (2002) Medical electrical equipment — part 2–44: Particular requirements for the basic safety and essential performance of X-ray equipment for computed tomography. IEC Publication No. 60601–2-44. Ed. 2.1, IEC Central Office, Geneva, Switzerland
The Leapfrog Group (2021) Leapfrog hospital survey. https://www.leapfroggroup.org/sites/default/files/Files/2021HospitalSurvey_20210913_v8.2%20%28version%203%29.pdf. Accessed 4 Oct 2021
Senate of the State of California (2010) Senate Bill No 1237. http://www.leginfo.ca.gov/pub/09-10/bill/sen/sb_1201-1250/sb_1237_bill_20100623_amended_asm_v97.pdf. Accessed 2 Oct 2021
Boone JM, Strauss KJ, Cody DD et al (2011) AAPM report no. 204. Size-specific dose estimates (SSDE) in pediatric and adult body CT examination. American Association of Physicists in Medicine, College Park, MD
McCollough C, Bakalyar DM, Bostani M et al (2014) Use of water equivalent diameter for calculating patient size and size-specific dose estimates (SSDE) in CT: the report of AAPM task group 220. American Association of Physicists in Medicine, College Park, MD
Kleinman PL, Strauss KJ, Zurakowski D et al (2010) Patient size measured on CT images as a function of age at a tertiary care children's hospital. AJR Am J Roentgenol 194:1611–1619
Acknowledgments
The authors acknowledge the generous funding of the U.S. National Institutes of Health and Patient-Centered Outcomes Research Institute. Funding sources played no role in study design, data collection, analysis, interpretation, reporting or decision to publish. We thank Carly Stewart and Susanna McIntyre for reviewing, editing and preparing the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Dr. Rebecca Smith-Bindman is the founder of Alara Imaging, a company focused on collecting and reporting radiation dose information associated with CT.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Chu, P.W., Yu, S., Wang, Y. et al. Reference phantom selection in pediatric computed tomography using data from a large, multicenter registry. Pediatr Radiol 52, 445–452 (2022). https://doi.org/10.1007/s00247-021-05227-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00247-021-05227-0