Abstract
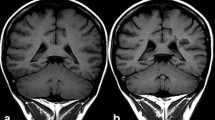
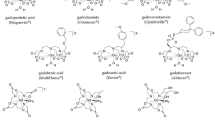
Gadolinium chelates have been used as standard contrast agents for clinical MRI for several decades. However, several investigators recently reported that rare Earth metals such as gadolinium are deposited in the brain for months or years. This is particularly concerning for children, whose developing brain is more vulnerable to exogenous toxins compared to adults. Therefore, a search is under way for alternative MR imaging biomarkers. The United States Food and Drug Administration (FDA)-approved iron supplement ferumoxytol can solve this unmet clinical need: ferumoxytol consists of iron oxide nanoparticles that can be detected with MRI and provide significant T1- and T2-signal enhancement of vessels and soft tissues. Several investigators including our research group have started to use ferumoxytol off-label as a new contrast agent for MRI. This article reviews the existing literature on the biodistribution of ferumoxytol in children and compares the diagnostic accuracy of ferumoxytol- and gadolinium-chelate-enhanced MRI. Iron oxide nanoparticles represent a promising new class of contrast agents for pediatric MRI that can be metabolized and are not deposited in the brain.






Similar content being viewed by others
References
Huguet M, Tobon-Gomez C, Bijnens BH et al (2009) Cardiac injuries in blunt chest trauma. J Cardiovasc Magn Reson 11:35
Huisman TA, Sorensen AG (2004) Perfusion-weighted magnetic resonance imaging of the brain: techniques and application in children. Eur Radiol 14:59–72
Kim RJ, Wu E, Rafael A et al (2000) The use of contrast-enhanced magnetic resonance imaging to identify reversible myocardial dysfunction. N Engl J Med 343:1445–1453
Rosenbaum DG, Askin G, Beneck DM et al (2017) Differentiating perforated from non-perforated appendicitis on contrast-enhanced magnetic resonance imaging. Pediatr Radiol 47:1483–1490
Sugimoto H, Takeda A, Hyodoh K (2001) MR imaging for evaluation of early rheumatoid arthritis. Semin Musculoskelet Radiol 5:159–165
Wakabayashi H, Saito J, Taki J et al (2016) Triple-phase contrast-enhanced MRI for the prediction of preoperative chemotherapeutic effect in patients with osteosarcoma: comparison with (99m)Tc-MIBI scintigraphy. Skelet Radiol 45:87–95
Simon G, Link TM, Wortler K et al (2005) Detection of hepatocellular carcinoma: comparison of Gd-DTPA- and ferumoxides-enhanced MR imaging. Eur Radiol 15:895–903
Clauser P, Helbich TH, Kapetas P et al (2019) Breast lesion detection and characterization with contrast-enhanced magnetic resonance imaging: prospective randomized intraindividual comparison of gadoterate meglumine (0.15 mmol/kg) and gadobenate dimeglumine (0.075 mmol/kg) at 3T. J Magn Reson Imaging 49:1157–1165
Lohrke J, Frenzel T, Endrikat J et al (2016) 25 years of contrast-enhanced MRI: developments, current challenges and future perspectives. Adv Ther 33:1–28
Mikati AG, Tan H, Shenkar R et al (2014) Dynamic permeability and quantitative susceptibility: related imaging biomarkers in cerebral cavernous malformations. Stroke 45:598–601
Daldrup-Link HE, Simon GH, Brasch RC (2006) Imaging of tumor angiogenesis: current approaches and future prospects. Curr Pharm Des 12:2661–2672
Zhou Z, Lu ZR (2012) Gadolinium-based contrast agents for magnetic resonance cancer imaging. Wiley Interdiscip Rev Nanomed Nanobiotechnol 5:1–18
Perazella MA (2009) Current status of gadolinium toxicity in patients with kidney disease. Clin J Am Soc Nephrol 4:461–469
Bennett CL, Qureshi ZP, Sartor AO et al (2012) Gadolinium-induced nephrogenic systemic fibrosis: the rise and fall of an iatrogenic disease. Clin Kidney J 5:82–88
Perazella MA (2009) Advanced kidney disease, gadolinium and nephrogenic systemic fibrosis: the perfect storm. Curr Opin Nephrol Hypertens 18:519–525
Thomsen HS, Morcos SK, Almen T et al (2013) Nephrogenic systemic fibrosis and gadolinium-based contrast media: updated ESUR contrast medium safety committee guidelines. Eur Radiol 23:307–318
McDonald RJ, McDonald JS, Kallmes DF et al (2015) Intracranial gadolinium deposition after contrast-enhanced MR imaging. Radiology 275:772–782
Kanda T, Matsuda M, Oba H et al (2015) Gadolinium deposition after contrast-enhanced MR imaging. Radiology 277:924–925
Guo BJ, Yang ZL, Zhang LJ (2018) Gadolinium deposition in brain: current scientific evidence and future perspectives. Front Mol Neurosci 11:335
Endrikat J, Dohanish S, Schleyer N et al (2018) 10 years of nephrogenic systemic fibrosis: a comprehensive analysis of nephrogenic systemic fibrosis reports received by a pharmaceutical company from 2006 to 2016. Investig Radiol 53:541–550
Sherry AD, Caravan P, Lenkinski RE (2009) Primer on gadolinium chemistry. J Magn Reson Imaging 30:1240–1248
Murata N, Gonzalez-Cuyar LF, Murata K et al (2016) Macrocyclic and other non-group 1 gadolinium contrast agents deposit low levels of gadolinium in brain and bone tissue: preliminary results from 9 patients with normal renal function. Investig Radiol 51:447–453
Roman-Goldstein SM, Barnett PA, McCormick CI et al (1991) Effects of gadopentetate dimeglumine administration after osmotic blood-brain barrier disruption: toxicity and MR imaging findings. AJNR Am J Neuroradiol 12:885–890
Ray DE, Cavanagh JB, Nolan CC et al (1996) Neurotoxic effects of gadopentetate dimeglumine: behavioral disturbance and morphology after intracerebroventricular injection in rats. AJNR Am J Neuroradiol 17:365–373
Miller JH, Hu HH, Pokorney A et al (2015) MRI brain signal intensity changes of a child during the course of 35 gadolinium contrast examinations. Pediatrics 136:e1637–e1640
Roberts DR, Holden KR (2016) Progressive increase of T1 signal intensity in the dentate nucleus and globus pallidus on unenhanced T1-weighted MR images in the pediatric brain exposed to multiple doses of gadolinium contrast. Brain Dev 38:331–336
Costa LG, Aschner M, Vitalone A et al (2004) Developmental neuropathology of environmental agents. Annu Rev Pharmacol Toxicol 44:87–110
Lanphear BP (2015) The impact of toxins on the developing brain. Annu Rev Public Health 36:211–230
Weinmann HJ, Brasch RC, Press WR et al (1984) Characteristics of gadolinium-DTPA complex: a potential NMR contrast agent. AJR Am J Roentgenol 142:619–624
Toth GB, Varallyay CG, Horvath A et al (2017) Current and potential imaging applications of ferumoxytol for magnetic resonance imaging. Kidney Int 92:47–66
Lu M, Cohen MH, Rieves D et al (2010) FDA report: ferumoxytol for intravenous iron therapy in adult patients with chronic kidney disease. Am J Hematol 85:315–319
Balakrishnan VS, Rao M, Kausz AT et al (2009) Physicochemical properties of ferumoxytol, a new intravenous iron preparation. Eur J Clin Investig 39:489–496
Schwenk MH (2010) Ferumoxytol: a new intravenous iron preparation for the treatment of iron deficiency anemia in patients with chronic kidney disease. Pharmacotherapy 30:70–79
Khurana A, Nejadnik H, Chapelin F et al (2013) Ferumoxytol: a new, clinically applicable label for stem-cell tracking in arthritic joints with MRI. Nanomedicine 8:1969–1983
Khurana A, Nejadnik H, Gawande R et al (2012) Intravenous ferumoxytol allows noninvasive MR imaging monitoring of macrophage migration into stem cell transplants. Radiology 264:803–811
Neuwelt EA, Varallyay CG, Manninger S et al (2007) The potential of ferumoxytol nanoparticle magnetic resonance imaging, perfusion, and angiography in central nervous system malignancy: a pilot study. Neurosurgery 60:601–611
Simon GH, von Vopelius-Feldt J, Fu Y et al (2006) Ultrasmall supraparamagnetic iron oxide-enhanced magnetic resonance imaging of antigen-induced arthritis: a comparative study between SHU 555 C, ferumoxtran-10, and ferumoxytol. Investig Radiol 41:45–51
Stabi KL, Bendz LM (2011) Ferumoxytol use as an intravenous contrast agent for magnetic resonance angiography. Ann Pharmacother 45:1571–1575
Singh A, Patel T, Hertel J et al (2008) Safety of ferumoxytol in patients with anemia and CKD. Am J Kidney Dis 52:907–915
Spinowitz BS, Kausz AT, Baptista J et al (2008) Ferumoxytol for treating iron deficiency anemia in CKD. J Am Soc Nephrol 19:1599–1605
Spinowitz BS, Schwenk MH, Jacobs PM et al (2005) The safety and efficacy of ferumoxytol therapy in anemic chronic kidney disease patients. Kidney Int 68:1801–1807
Iv M, Choudhri O, Dodd RL et al (2018) High-resolution 3D volumetric contrast-enhanced MR angiography with a blood pool agent (ferumoxytol) for diagnostic evaluation of pediatric brain arteriovenous malformations. J Neurosurg Pediatr 22:251–260
Lai LM, Cheng JY, Alley MT et al (2017) Feasibility of ferumoxytol-enhanced neonatal and young infant cardiac MRI without general anesthesia. J Magn Reson Imaging 45:1407–1418
Muehe AM, Theruvath AJ, Lai L et al (2018) How to provide gadolinium-free PET/MR cancer staging of children and young adults in less than 1 h: the Stanford approach. Mol Imaging Biol 20:324–335
Mohanty S, Chen Z, Li K et al (2017) A novel theranostic strategy for MMP-14-expressing glioblastomas impacts survival. Mol Cancer Ther 16:1909–1921
Zanganeh S, Hutter G, Spitler R et al (2016) Iron oxide nanoparticles inhibit tumour growth by inducing pro-inflammatory macrophage polarization in tumour tissues. Nat Nanotechnol 11:986–994
Nejadnik H, Lenkov O, Gassert F et al (2016) Macrophage phagocytosis alters the MRI signal of ferumoxytol-labeled mesenchymal stromal cells in cartilage defects. Sci Rep 6:25897
Iv M, Telischak N, Feng D et al (2015) Clinical applications of iron oxide nanoparticles for magnetic resonance imaging of brain tumors. Nanomedicine 10:993–1018
Shi Q, Pisani LJ, Lee YK et al (2013) Evaluation of the novel USPIO GEH121333 for MR imaging of cancer immune responses. Contrast Media Mol Imaging 8:281–288
Daldrup-Link HE, Mohanty A, Cuenod C et al (2009) New perspectives on bone marrow contrast agents and molecular imaging. Semin Musculoskelet Radiol 13:145–156
Daldrup-Link HE, Henning T, Link TM (2007) MR imaging of therapy-induced changes of bone marrow. Eur Radiol 17:743–761
Metz S, Lohr S, Settles M et al (2006) Ferumoxtran-10-enhanced MR imaging of the bone marrow before and after conditioning therapy in patients with non-Hodgkin lymphomas. Eur Radiol 16:598–607
Daldrup-Link HE, Rydland J, Helbich TH et al (2003) Quantification of breast tumor microvascular permeability with feruglose-enhanced MR imaging: initial phase II multicenter trial. Radiology 229:885–892
Patsialou A, Wyckoff J, Wang Y et al (2009) Invasion of human breast cancer cells in vivo requires both paracrine and autocrine loops involving the colony-stimulating factor-1 receptor. Cancer Res 69:9498–9506
Daldrup-Link H (2017) 10 things you might not know about iron oxide nanoparticles. Radiology 284:616–629
Hassan N, Cahill J, Rajasekaran S et al (2011) Ferumoxytol infusion in pediatric patients with gastrointestinal disorders: first case series. Ann Pharmacother 45:e63
Muehe AM, Feng D, von Eyben R et al (2016) Safety report of ferumoxytol for magnetic resonance imaging in children and young adults. Investig Radiol 51:221–227
Theruvath AJ, Aghighi M, Iv M et al (2020) Brain iron deposition after ferumoxytol-enhanced MRI: a study of porcine brains. Nanotheranostics 4:195–200
Iv M, Ng NN, Nair S et al (2020) Brain iron assessment after ferumoxytol-enhanced MRI in children and young adults with arteriovenous malformations: a case-control study. Radiology 297:438–446
Klenk C, Gawande R, Uslu L et al (2014) Ionising radiation-free whole-body MRI versus (18)F-fluorodeoxyglucose PET/CT scans for children and young adults with cancer: a prospective, non-randomised, single-Centre study. Lancet Oncol 15:275–285
Storey P, Lim RP, Chandarana H et al (2012) MRI assessment of hepatic iron clearance rates after USPIO administration in healthy adults. Investig Radiol 47:717–724
Barajas RF Jr, Hamilton BE, Schwartz D et al (2018) Combined iron oxide nanoparticle ferumoxytol and gadolinium contrast enhanced MRI defines glioblastoma pseudo-progression. Neuro Oncol 21:517–526
Li W, Tutton S, Vu AT et al (2005) First-pass contrast-enhanced magnetic resonance angiography in humans using ferumoxytol, a novel ultrasmall superparamagnetic iron oxide (USPIO)-based blood pool agent. J Magn Reson Imaging 21:46–52
Dosa E, Tuladhar S, Muldoon LL et al (2011) MRI using ferumoxytol improves the visualization of central nervous system vascular malformations. Stroke 42:1581–1588
Pohlmann A, Karczewski P, Ku MC et al (2014) Cerebral blood volume estimation by ferumoxytol-enhanced steady-state MRI at 9.4 T reveals microvascular impact of alpha1 -adrenergic receptor antibodies. NMR Biomed 27:1085–1093
Li W, Salanitri J, Tutton S et al (2007) Lower extremity deep venous thrombosis: evaluation with ferumoxytol-enhanced MR imaging and dual-contrast mechanism —preliminary experience. Radiology 242:873–881
Hamilton BE, Woltjer RL, Prola-Netto J et al (2016) Ferumoxytol-enhanced MRI differentiation of meningioma from dural metastases: a pilot study with immunohistochemical observations. J Neurooncol 129:301–309
Daldrup-Link HE, Kaiser A, Helbich T et al (2003) Macromolecular contrast medium (feruglose) versus small molecular contrast medium (gadopentetate) enhanced magnetic resonance imaging: differentiation of benign and malignant breast lesions. Acad Radiol 10:1237–1246
Vogl TJ, Hammerstingl R, Schwarz W et al (1996) Magnetic resonance imaging of focal liver lesions. Comparison of the superparamagnetic iron oxide resovist versus gadolinium-DTPA in the same patient. Investig Radiol 31:696–708
Lutz AM, Willmann JK, Goepfert K et al (2005) Hepatocellular carcinoma in cirrhosis: enhancement patterns at dynamic gadolinium- and superparamagnetic iron oxide-enhanced T1-weighted MR imaging. Radiology 237:520–528
Heilmaier C, Lutz AM, Bolog N et al (2009) Focal liver lesions: detection and characterization at double-contrast liver MR imaging with ferucarbotran and gadobutrol versus single-contrast liver MR imaging. Radiology 253:724–733
Siedek F, Muehe AM, Theruvath AJ et al (2020) Comparison of ferumoxytol and Gd-chelate-enhanced MRI for assessment of bone and soft tissue sarcomas in children and young adults. Eur Radiol 30:1790–1803
Muehe AM, Siedek F, Theruvath AJ et al (2020) Differentiation of benign and malignant lymph nodes in pediatric patients on ferumoxytol-enhanced PET/MRI. Theranostics 10:3612–3621
Daldrup-Link HE, Rummeny EJ, Ihssen B et al (2002) Iron-oxide-enhanced MR imaging of bone marrow in patients with non-Hodgkin's lymphoma: differentiation between tumor infiltration and hypercellular bone marrow. Eur Radiol 12:1557–1566
Ward J, Guthrie JA, Scott DJ et al (2000) Hepatocellular carcinoma in the cirrhotic liver: double-contrast MR imaging for diagnosis. Radiology 216:154–162
Gahramanov S, Raslan AM, Muldoon LL et al (2010) Potential for differentiation of pseudoprogression from true tumor progression with dynamic susceptibility-weighted contrast-enhanced magnetic resonance imaging using ferumoxytol vs. gadoteridol: a pilot study. Int J Radiat Oncol Biol Phys 79:514–523
Daldrup-Link HE, Golovko D, Ruffel B et al (2011) MR imaging of tumor associated macrophages with clinically-applicable iron oxide nanoparticles. Clin Cancer Res 17:5695–5704
Aghighi M, Theruvath AJ, Pareek A et al (2018) Magnetic resonance imaging of tumor-associated macrophages: clinical translation. Clin Cancer Res 24:4110–4118
Iv M, Samghabadi P, Holdsworth S et al (2019) Quantification of macrophages in high-grade gliomas by using ferumoxytol-enhanced MRI: a pilot study. Radiology 290:198–206
Mohanty S, Aghighi M, Yerneni K et al (2019) Improving the efficacy of osteosarcoma therapy: combining drugs that turn cancer cell 'don't eat me' signals off and 'eat me' signals on. Mol Oncol 13:2049–2061
Mohanty S, Yerneni K, Graef CM et al (2018) Imaging therapy response of osteosarcoma to anti-CD47 therapy. Cell Death Dis 10:36–49
Aghighi M, Golovko D, Ansari C et al (2015) Imaging tumor necrosis with ferumoxytol. PLoS One 10:e0142665
Lim HS, Jeong YY, Kang HK et al (2006) Imaging features of hepatocellular carcinoma after transcatheter arterial chemoembolization and radiofrequency ablation. AJR Am J Roentgenol 187:W341–W349
Fukumura D, Duda DG, Munn LL et al (2010) Tumor microvasculature and microenvironment: novel insights through intravital imaging in pre-clinical models. Microcirculation 17:206–225
Simon GH, Bauer J, Saborovski O et al (2006) T1 and T2 relaxivity of intracellular and extracellular USPIO at 1.5 T and 3T clinical MR scanning. Eur Radiol 16:738–745
Turetschek K, Huber S, Floyd E et al (2001) MR imaging characterization of microvessels in experimental breast tumors by using a particulate contrast agent with histopathologic correlation 1. Radiology 218:562–569
Hanna RF, Kased N, Kwan SW et al (2008) Double-contrast MRI for accurate staging of hepatocellular carcinoma in patients with cirrhosis. AJR Am J Roentgenol 190:47–57
Guiu B, Loffroy R, Ben Salem D et al (2008) Combined SPIO-gadolinium magnetic resonance imaging in cirrhotic patients: negative predictive value and role in screening for hepatocellular carcinoma. Abdom Imaging 33:520–528
Daldrup-Link HE, Rudelius M, Piontek G et al (2005) Migration of iron oxide-labeled human hematopoietic progenitor cells in a mouse model: in vivo monitoring with 1.5-T MR imaging equipment. Radiology 234:197–205
Simon GH, von Vopelius-Feldt J, Wendland MF et al (2006) MRI of arthritis: comparison of ultrasmall superparamagnetic iron oxide vs. Gd-DTPA. J Magn Reson Imaging 23:720–727
Henning TD, Sutton EJ, Kim A et al (2009) The influence of ferucarbotran on the chondrogenesis of human mesenchymal stem cells. Contrast Media Mol Imaging 4:165–173
Henning TD, Wendland MF, Golovko D et al (2009) Relaxation effects of ferucarbotran-labeled mesenchymal stem cells at 1.5T and 3T: discrimination of viable from lysed cells. Magn Reson Med 62:325–332
Nedopil A, Klenk C, Kim C et al (2010) MR signal characteristics of viable and apoptotic human mesenchymal stem cells in matrix-associated stem cell implants for treatment of osteoarthritis. Investig Radiol 45:634–640
Meier R, Golovko D, Tavri S et al (2011) Depicting adoptive immunotherapy for prostate cancer in an animal model with magnetic resonance imaging. Magn Reson Med 65:756–763
Henning TD, Gawande R, Khurana A et al (2012) Magnetic resonance imaging of ferumoxide-labeled mesenchymal stem cells in cartilage defects: in vitro and in vivo investigations. Mol Imaging 11:197–209
Khurana A, Chapelin F, Beck G et al (2013) Iron administration before stem cell harvest enables MR imaging tracking after transplantation. Radiology 269:186–197
Ansari C, Tikhomirov GA, Hong SH et al (2014) Cancer therapy: development of novel tumor-targeted theranostic nanoparticles activated by membrane-type matrix metalloproteinases for combined cancer magnetic resonance imaging and therapy. Small 10:566–575, 417
Zhu J, Zhou L, XingWu F (2006) Tracking neural stem cells in patients with brain trauma. N Engl J Med 355:2376–2378
Callera F, de Melo CM (2007) Magnetic resonance tracking of magnetically labeled autologous bone marrow CD34+ cells transplanted into the spinal cord via lumbar puncture technique in patients with chronic spinal cord injury: CD34+ cells' migration into the injured site. Stem Cells Dev 16:461–466
de Vries IJ, Lesterhuis WJ, Barentsz JO et al (2005) Magnetic resonance tracking of dendritic cells in melanoma patients for monitoring of cellular therapy. Nat Biotechnol 23:1407–1413
Toso C, Vallee JP, Morel P et al (2008) Clinical magnetic resonance imaging of pancreatic islet grafts after iron nanoparticle labeling. Am J Transplant 8:701–706
Theruvath AJ, Nejadnik H, Muehe AM et al (2018) Tracking cell transplants in femoral osteonecrosis with magnetic resonance imaging: a proof-of-concept study in patients. Clin Cancer Res 24:6223–6229
McEnery PT, Stablein DM, Arbus G et al (1992) Renal transplantation in children. A report of the North American Pediatric Renal Transplant Cooperative Study. N Engl J Med 326:1727–1732
Birk PE (2012) Surveillance biopsies in children post-kidney transplant. Pediatr Nephrol 27:753–760
Aghighi M, Pisani L, Theruvath AJ et al (2017) Ferumoxytol is not retained in kidney allografts in patients undergoing acute rejection. Mol Imaging Biol 20:139–149
Kriz J, Jirák D, Girman P et al (2005) Magnetic resonance imaging of pancreatic islets in tolerance and rejection. Transplantation 80:1596–1603
Evgenov NV, Medarova Z, Pratt J et al (2006) In vivo imaging of immune rejection in transplanted pancreatic islets. Diabetes 55:2419–2428
Chae EY, Song EJ, Sohn JY et al (2010) Allogeneic renal graft rejection in a rat model: in vivo MR imaging of the homing trait of macrophages 1. Radiology 256:847–854
Hauger O, Grenier N, Deminère C et al (2007) USPIO-enhanced MR imaging of macrophage infiltration in native and transplanted kidneys: initial results in humans. Eur Radiol 17:2898–2907
Ricardo SD, van Goor H, Eddy AA (2008) Macrophage diversity in renal injury and repair. J Clin Invest 118:3522–3530
Magil AB (2009) Monocytes/macrophages in renal allograft rejection. Transplant Rev 23:199–208
Tinckam KJ, Djurdjev O, Magil AB (2005) Glomerular monocytes predict worse outcomes after acute renal allograft rejection independent of C4d status. Kidney Int 68:1866–1874
Thoeny HC, Zumstein D, Simon-Zoula S et al (2006) Functional evaluation of transplanted kidneys with diffusion-weighted and BOLD MR imaging: initial experience 1. Radiology 241:812–821
Sadowski EA, Djamali A, Wentland AL et al (2010) Blood oxygen level-dependent and perfusion magnetic resonance imaging: detecting differences in oxygen bioavailability and blood flow in transplanted kidneys. Magn Reson Imaging 28:56–64
Wentland AL, Sadowski EA, Djamali A et al (2009) Quantitative MR measures of intrarenal perfusion in the assessment of transplanted kidneys: initial experience. Acad Radiol 16:1077–1085
Khan S, Amin FM, Fliedner FP et al (2019) Investigating macrophage-mediated inflammation in migraine using ultrasmall superparamagnetic iron oxide-enhanced 3T magnetic resonance imaging. Cephalalgia 39:1407–1420
Acknowledgments
This study was supported by a grant from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD, R01 HD081123A) and the National Institute of Arthritis and Musculoskeletal and Skin Diseases (R01AR054458).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
None
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Daldrup-Link, H.E., Theruvath, A.J., Rashidi, A. et al. How to stop using gadolinium chelates for magnetic resonance imaging: clinical-translational experiences with ferumoxytol. Pediatr Radiol 52, 354–366 (2022). https://doi.org/10.1007/s00247-021-05098-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00247-021-05098-5