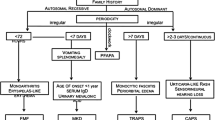
Abstract
Autoinflammatory diseases constitute a family of disorders defined by aberrant stimulation of inflammatory pathways without involving antigen-directed autoimmunity. They may be divided into monogenic and polygenic types. Monogenic autoinflammatory syndromes are those with identified genetic mutations, such as familial Mediterranean fever, tumor necrosis factor receptor-associated periodic fever syndrome (TRAPS), mevalonate kinase deficiency or hyperimmunoglobulin D syndrome, cryopyrin-associated periodic fever syndromes (CAPS), pyogenic arthritis pyoderma gangrenosum and acne (PAPA) syndrome, interleukin-10 and interleukin-10 receptor deficiencies, adenosine deaminase 2 deficiency and pediatric sarcoidosis. Those without an identified genetic mutation are known as polygenic and include systemic-onset juvenile idiopathic arthritis, idiopathic recurrent acute pericarditis, Behçet syndrome, chronic recurrent multifocal osteomyelitis and inflammatory bowel disease among others. Autoinflammatory disorders are defined by repeating episodes or persistent fever, rash, serositis, lymphadenopathy, arthritis and increased acute phase reactants, and thus may mimic infections clinically. Most monogenic autoinflammatory syndromes present in childhood. However, because of their infrequency, diverse and nonspecific presentation, and the relatively new genetic recognition, diagnosis is usually delayed. In this article, which is Part 1 of a two-part series, the authors update monogenic autoinflammatory diseases in children with special emphasis on imaging features that may help establish the correct diagnosis.














Similar content being viewed by others
References
McDermott MF, Aksentijevich I, Galon J et al (1999) Germline mutations in the extracellular domains of the 55 kda TNF receptor, TNFR1, define a family of dominantly inherited autoinflammatory syndromes. Cell 97:133–144
Almeida de Jesus A, Goldbach-Mansky R (2013) Monogenic autoinflammatory diseases: concept and clinical manifestations. Clin Immunol 147:155–174
Masters SL, Simon A, Aksentijevich I, Kastner DL (2009) Horror autoinflammaticus: the molecular pathophysiology of autoinflammatory disease. Annu Rev Immunol 27:621–668
Barron KS, Kastner DL (2016) Periodic fever syndromes and other inherited autoinflammatory diseases. In: Petty RE, Laxer RM, Lindsley CB, Wedderburn LR (eds) Textbook of pediatric rheumatology, 7th edn. Elsevier, Philadelphia, pp 609–626
French FMF Consortium (1997) A candidate gene for familial Mediterranean fever. Nat Genet 17:25–31
Gershoni-Baruch R, Shinawi M, Leah K et al (2001) Familial Mediterranean fever: prevalence, penetrance and genetic drift. Eur J Hum Genet 9:634–637
Zissin R, Rathaus V, Gayer G et al (2003) CT findings in patients with familial Mediterranean fever during an acute abdominal attack. Br J Radiol 76:22–25
Aharoni D, Hiller N, Hadas-Halpern I (2000) Familial Mediterranean fever: abdominal imaging findings in 139 patients and review of the literature. Abdom Imaging 25:297–300
De Socio G, Cerquaglia C, Curigliano V et al (2009) Association between familial mediterranean fever and retroperitoneal fibrosis: retroperitoneal fibrosis regression after colchicine therapy. Int J Immunopathol Pharmacol 22:521–524
Gurkan OE, Dalgic B (2013) Gastrointestinal mucosal involvement without amyloidosis in children with familial Mediterranean fever. J Pediatr Gastroenterol Nutr 57:319–323
Ishak GE, Khoury NJ, Birjawi GA et al (2006) Imaging findings of familial Mediterranean fever. Clin Imaging 30:153–159
Kees S, Langevitz P, Zemer D et al (1997) Attacks of pericarditis as a manifestation of familial Mediterranean fever (FMF). QJM 90:643–647
Ince E, Cakar N, Tekin M et al (2002) Arthritis in children with familial Mediterranean fever. Rheumatol Int 21:213–217
Uthman I, Hajj-Ali RA, Arayssi T et al (2001) Arthritis in familial Mediterranean fever. Rheumatol Int 20:145–148
Brodey PA, Wolff SM (1975) Radiographic changes in the sacroiliac joints in familial Mediterranean fever. Radiology 114:331–333
Makay BB, Kefi A, Ünsal E (2007) Familial Mediterranean fever in the differential diagnosis of pediatric acute scrotum. Ege J Med 46:101–103
Gedalia A, Adar A, Gorodischer R (1992) Familial Mediterranean fever in children. J Rheumatol Suppl 35:1–9
Hull KM, Drewe E, Aksentijevich I et al (2002) The TNF receptor-associated periodic syndrome (TRAPS): emerging concepts of an autoinflammatory disorder. Medicine (Baltimore) 81:349–368
Chen YJ, Yu HH, Yang YH et al (2014) Recurrent abdominal pain as the presentation of tumor necrosis factor receptor-associated periodic syndrome (TRAPS) in an Asian girl: a case report and review of the literature. J Microbiol Immunol Infect 47:550–554
Alvarez-Lobos M, Hunter B, Cofré C et al (2006) Tumor necrosis factor receptor associated periodic syndrome (TRAPS). Report of two cases. Rev Med Chil 134:1558–1561
Stankovic K, Grateau G (2007) Auto inflammatory syndromes: diagnosis and treatment. Joint Bone Spine 74:544–550
Cantarini L, Lucherini OM, Cimaz R et al (2010) Sacroileitis and pericarditis: atypical presentation of tumor necrosis factor receptor-associated periodic syndrome and response to etanercept therapy. Clin Exp Rheumatol 28:290–291
Hull KM, Wong K, Wood GM et al (2002) Monocytic fasciitis: a newly recognized clinical feature of tumor necrosis factor receptor dysfunction. Arthritis Rheum 46:2189–2194
Dodé C, Papo T, Fieschi C (2000) A novel missense mutation (C30S) in the gene encoding tumor necrosis factor receptor 1 linked to autosomal-dominant recurrent fever with localized myositis in a French family. Arthritis Rheum 43:1535–1542
Goldstein JL, Brown MS (1990) Regulation of the mevalonate pathway. Nature 343:425–430
Drenth JP, Cuisset L, Grateau G et al (1999) Mutations in the gene encoding mevalonate kinase cause hyper-IgD and periodic fever syndrome. International hyper-IgD study group. Nat Genet 22:178–1781
Mulders-Manders CM, Simon A (2015) Hyper-IgD syndrome/mevalonate kinase deficiency: what is new? Semin Immunopathol 37:371–376
van der Hilst JC, Bodar EJ, Barron KS et al (2008) Long-term follow-up, clinical features, and quality of life in a series of 103 patients with hyperimmunoglobulinemia D syndrome. Medicine (Baltimore) 87:301–310
Haas D, Hoffmann GF (2006) Mevalonate kinase deficiencies: from mevalonic aciduria to hyperimmunoglobulinemia D syndrome. Orphanet J Rare Dis 1:13
Osborn RE, Alder DC, Mitchell CS (1991) MR imaging of the brain in patients with migraine headaches. AJNR Am J Neuroradiol 12:521–524
Ruiz Gomez A, Couce ML, Garcia-Villoria J et al (2012) Clinical, genetic, and therapeutic diversity in 2 patients with severe mevalonate kinase deficiency. Pediatrics 129:e535–e539
Bretón Martínez JR, Cánovas Martínez A, Casaña Pérez S et al (2007) Mevalonic aciduria: report of two cases. J Inherit Metab Dis 30:829
Galeotti C, Meinzer U, Quartier P et al (2012) Efficacy of interleukin-1-targeting drugs in mevalonate kinase deficiency. Rheumatology (Oxford) 51:1855–1859
Stahl N, Radin A, Mellis S (2009) Rilonacept--CAPS and beyond. Ann N Y Acad Sci 1182:124–134
Hoffman HM, Wanderer AA, Broide DH (2001) Familial cold autoinflammatory syndrome: phenotype and genotype of an autosomal dominant periodic fever. J Allergy Clin Immunol 108:615–620
Neven B, Prieur AM, Quartier dit Maire P (2008) Cryopyrinopathies: update on pathogenesis and treatment. Nat Clin Pract Rheumatol 4:481–489
Compeyrot-Lacassagne S, Tran TA, Guillaume-Czitrom S et al (2009) Brain multiple sclerosis-like lesions in a patient with muckle-Wells syndrome. Rheumatology (Oxford) 48:1618–1619
Dávila-Seijo P, Hernández-Martín A, Torrelo A (2014) Autoinflammatory syndromes for the dermatologist. Clin Dermatol 32:488–501
Morbach H, Hedrich CM, Beer M, Girschick HJ (2013) Autoinflammatory bone disorders. Clin Immunol 147:185–196
Zaki FM, Sridharan R, Pei TS et al (2012) NOMID: the radiographic and MRI features and review of literature. J Radiol Case Rep 6:1–8
Torbiak RP, Dent PB, Cockshott WP (1989) NOMID – a neonatal syndrome of multisystem inflammation. Skeletal Radiol 18:359–364
Hill SC, Namde M, Dwyer A et al (2007) Arthropathy of neonatal onset multisystem inflammatory disease (NOMID/CINCA). Pediatr Radiol 37:145–152
Goldbach-Mansky R, Dailey NJ, Canna SW et al (2006) Neonatal-onset multisystem inflammatory disease responsive to interleukin-1beta inhibition. N Engl J Med 355:581–592
Lequerré T, Vittecoq O, Saugier-Veber P et al (2007) A cryopyrin-associated periodic syndrome with joint destruction. Rheumatology (Oxford) 46:709–714
Neven B, Marvillet I, Terrada C et al (2010) Long-term efficacy of the interleukin-1 receptor antagonist anakinra in ten patients with neonatal-onset multisystem inflammatory disease/chronic infantile neurologic, cutaneous, articular syndrome. Arthritis Rheum 62:258–267
Smith EJ, Allantaz F, Bennett L et al (2010) Clinical, molecular, and genetic characteristics of PAPA syndrome: a review. Curr Genomics 11:519–527
Martinez-Rios C, Jariwala MP, Highmore K et al (2019) Imaging findings of sterile pyogenic arthritis, pyoderma gangrenosum and acne (PAPA) syndrome: differential diagnosis and review of the literature. Pediatr Radiol 49:23–36
Lindor NM, Arsenault TM, Solomon H et al (1997) A new autosomal dominant disorder of pyogenic sterile arthritis, pyoderma gangrenosum, and acne: PAPA syndrome. Mayo Clin Proc 72:611–615
Caorsi R, Picco P, Buoncompagni A et al (2014) Osteolytic lesion in PAPA syndrome responding to anti-interleukin 1 treatment. J Rheumatol 41:2333–2334
Demidowich AP, Freeman AF, Kuhns DB et al (2012) Brief report: genotype, phenotype, and clinical course in five patients with PAPA syndrome (pyogenic sterile arthritis, pyoderma gangrenosum, and acne). Arthritis Rheum 64:2022–2027
Engelhardt KR, Shah N, Faizura-Yeop I et al (2013) Clinical outcome in IL-10- and IL-10 receptor-deficient patients with or without hematopoietic stem cell transplantation. J Allergy Clin Immunol 131:825–830
Glocker EO, Kotlarz D, Boztug K et al (2009) Inflammatory bowel disease and mutations affecting the interleukin-10 receptor. N Engl J Med 361:2033–2045
Kotlarz D, Beier R, Murugan D et al (2012) Loss of interleukin-10 signaling and infantile inflammatory bowel disease: implications for diagnosis and therapy. Gastroenterology 143:347–355
Kuşkonmaz B, Ayvaz D, Aydemir Y et al (2016) Successful outcome with second hematopoietic stem cell transplantation in a patient with IL-10R deficiency. Bone Marrow Transplant 51:615–616
Zhou Q, Yang D, Ombrello AK et al (2014) Early-onset stroke and vasculopathy associated with mutations in ADA2. N Engl J Med 370:911–920
Sahin S, Adrovic A, Barut K et al (2018) Clinical, imaging and genotypical features of three deceased and five surviving cases with ADA2 deficiency. Rheumatol Int 38:129–136
Elbracht M, Mull M, Wagner N et al (2017) Stroke as initial manifestation of adenosine deaminase 2 deficiency. Neuropediatrics 48:111–114
Bulut E, Erden A, Karadag O et al (2019) Deficiency of adenosine deaminase 2; special focus on central nervous system imaging. J Neuroradiol 46:193–198
Mirsky DM, Beslow LA, Amlie-Lefond C et al (2017) Pathways for neuroimaging of childhood stroke. Pediatr Neurol 69:11–23
Adler Y, Charron P, Imazio M et al (2015) ESC guidelines for the diagnosis and management of pericardial diseases. Rev Esp Cardiol (Engl Ed) 68:1126
Aróstegui JI, Arnal C, Merino R et al (2007) NOD2 gene-associated pediatric granulomatous arthritis: clinical diversity, novel and recurrent mutations, and evidence of clinical improvement with interleukin-1 blockade in a Spanish cohort. Arthritis Rheum 56:3805–3813
Rosé CD, Wouters CH, Meiorin S et al (2006) Pediatric granulomatous arthritis: an international registry. Arthritis Rheum 54:3337–3344
Ikeda K, Kambe N, Satoh T et al (2013) Preferentially inflamed tendon sheaths in the swollen but not tender joints in a 5-year-old boy with Blau syndrome. J Pediatr 163:1525.e1
Rosé CD, Wouters C (2016) Pediatric sarcoidosis. In: Petty RE, Laxer RM, Lindsley CB, Wedderburn LR (eds) Textbook of pediatric rheumatology, 7th edn. Elsevier, Philadelphia, pp 517–525
Rosé CD, Pans S, Casteels I et al (2015) Blau syndrome: cross-sectional data from a multicentre study of clinical, radiological and functional outcomes. Rheumatology (Oxford) 54:1008–1016
Ikeda K, Kambe N, Takei S et al (2014) Ultrasonographic assessment reveals detailed distribution of synovial inflammation in Blau syndrome. Arthritis Res Ther 16:R89
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
None
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Navallas, M., Inarejos Clemente, E.J., Iglesias, E. et al. Autoinflammatory diseases in childhood, part 1: monogenic syndromes. Pediatr Radiol 50, 415–430 (2020). https://doi.org/10.1007/s00247-019-04536-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00247-019-04536-9